Purification and Partial Characterization of a Coagulant Tle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

9Th & 10Th Grade
1/27/2019 SpeechWire Tournament Services Ridge Invitational Declamation (9th & 10th Grade) Elimination round results Final round All Judges Code Name Team LK1 WK1 AA1 All ranks Place recips pref. AH101 Niamh Campbell Phoenix Country Day School 1 1 6 14 6.67 1 LP103 Matthew Floyd Catholic Memorial 2 2 1 15 5.25 2 BD109 Ashin Varghese Randolph High School 3 3 2 16 5.42 3 AO121 Juliette Gringeri Summit High School 5 4 4 23 3.95 4 UT102 Farrah Culmer Democracy Prep Harlem Prep 7 6 3 24 4.48 5 AH108 Enzo Acharya Phoenix Country Day School 6 5 7 26 4.01 6 AG101 Brynn Nelson Fontbonne Hall Academy 4 7 5 26 3.84 7 Semifinal round All Judges Code Name Team AP2 BD1 All ranks Place recips pref. AH101 Niamh Campbell Phoenix Country Day School 1 1 6 4.50 1 BD109 Ashin Varghese Randolph High School 8 4.25 2 UT102 Farrah Culmer Democracy Prep Harlem Prep 8 3.83 3 AH108 Enzo Acharya Phoenix Country Day School 8 3.50 4 AG101 Brynn Nelson Fontbonne Hall Academy 10 3.25 5 AO121 Juliette Gringeri Summit High School 10 3.25 5 LP103 Matthew Floyd Catholic Memorial 2 2 10 3.25 5 AG102 Juliann Bianco Fontbonne Hall Academy 11 3.53 8 AL101 Adrian Tejada Democracy Prep Endurance High 11 3.00 9 AH112 Milan Sewell Phoenix Country Day School 12 2.58 10 BC115 Maria Chabanov Xaverian HS 13 2.08 11 AH109 Devan Amin Phoenix Country Day School 14 2.37 12 GG105 Uswad Qureshi Phillipsburg HS 14 2.33 13 AC107 Nina Li Strath Haven High School 3 4 14 1.92 14 BC116 Amy Gruber Xaverian HS 15 2.70 15 AH133 Ella Brenes Phoenix Country Day School 4 5 15 2.28 16 AM101 Jaina Jallow Livingston High School 5 3 15 1.87 17 AK108 Fatimata Ly Achievement First 17 2.60 18 CZ112 Rajit Sharma Union Catholic 17 2.03 19 Semifinal round All Judges Code Name Team AA5 RY3 All ranks Place recips pref. -

Plan Local D'urbanisme
d ép a rt em AB15 e n ta le AB18 AB135 AB167 AB168 AB133 AB14 AB137 AB19 510 000 511 000 AB131 512 000 513 000 514 000 515 000 AB159 R AB27 u i AB20 464 000 465 000 s 466 000 467 000 468 000 469 000 AB134 s e A a AB21 n AB127 u ci en AB169 AB28 AB24 AB23 n°1 AB156 AB157 29 AB29 Ch AB130 AB138 AB158 s em er i AB22 av n Tr AB139 AB126 AB25 AB26 AB140 AB196 u AB142 d de AB145 A AB141 AB42 AB43 AB34 AB125 AB129 in N d m AB32 e he AB143 C Sim AB128 d or e AB35 re é Techen AB155 AB36 du AB124 AB61 u uissea R LA BORDENEUVE-OUEST AB144 AB44 AB1 AB189 AB37 AB60 à AB40 Ro ute AB147 AB11 AB64 N AB38 AB4 AB123 AB146 AB45 AB65 Sei ssa AB2 AB188 AB49 n AB46 AB50 AB10 AB9 AB39 AB59 AB151 AB62 AB63 AB122 Anc AB3 AB152 iednép CRABOTS-EST art eme AB187 S nta AB121 e le is sa AB66 n L Ro es ut te AB148 e ou AB58 AB12 AB5 é AB120 AB52 ch s AB164 em r in n 1 e 29 Av B179 AC118 a AB13 AB119 r Do AC119 T AB57 n-Gr abot AB149 Nag AB76 AB150 AB6 AB118 AB178 AC120 N AB67 CRABOTS-OUEST AC121 de AB17 de AB136 AB117 AB177 AB8 AB115 A Ruisseau AB160 AB56 AB68 AB16 AB165 AB132 S AC123 AB55 e 0 AB116 is d s a 0 ép à n a rt 0 em AB15 AB180 en 5 ta l 6 e AB18 AB54 AB192 AC125 AB114 u AC122 2 AB166 AB69 AB168 d a AB167 AB135 0 AB173 AB133 6 AB14 0 AB113 Ruis seau 0 S i AB112 AB194 m AB193 5 AB137 AB19 o Nh CAILLAOUAS-NORD r r 6 L AB70 'Is e le-en 2 AB109 AB175 -Dod AC117 don N AB131 on AB110 AB159 AC124 6 R AB27 u i AB20 s AB134 s e AB21 A AB181 à a nc AB162 R. -

Integration of Myofibrils in the Developing Heart and Challenges on the Intercalated Disc Stability
Research Collection Doctoral Thesis Integration of myofibrils in the developing heart and challenges on the intercalated disc stability Author(s): Hirschy, Alain Publication Date: 2004 Permanent Link: https://doi.org/10.3929/ethz-a-005001295 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Dissertation ETH N°15790 Integration of myofibrils in the developing heart and challenges on the intercalated disc stability A dissertation submitted to the SWISS INSTITUTE OF TECHNOLOGY ZURICH (ETHZ) For the degree of Doctor of Natural Sciences presented by Alain Hirschy Biologiste diplômé (Université de Neuchâtel, Switzerland) Born April 15, 1975 Citizen of Neuchâtel Accepted on the recommendation of Prof. Dr Jean-Claude Perriard, examiner Prof. Dr Lukas Sommer, co-examiner Prof. Dr Thierry Pedrazzini, co-examiner November 2004 Table of contents Table of contents I Abbreviations IV Abstract 1 Résumé 3 1 Introduction 5 1.1 The heart 5 1.1.1 Morphological development of the heart 5 1.1.2 Molecular pathways controlling heart development 6 1.1.3 Development of ventricular cardiomyocytes 6 1.2 Myofibrillogenesis and development of cell-cell contacts in the ventricular myocardium 7 1.2.1 The contractile apparatus 7 1.2.2 Assembly of sarcomeric proteins 7 1.2.3 Development of cell-cell contacts 9 1.2.4 Three types of cell-cell contact form the intercalated disc 10 1.2.5 The adherens junction -
Childhood Asthma Management Program Data Dictionary
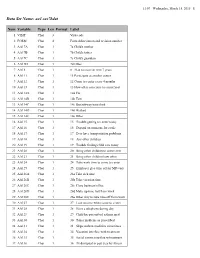
11:07 Wednesday, March 18, 2015 1 Data Set Name: aa1.sas7bdat Num Variable Type Len Format Label 1 VISIT Char 3 Visit code 2 FORM Char 4 Form abbreviation and revision number 3 AA17A Char 1 7a Child's mother 4 AA17B Char 1 7b Child's father 5 AA17C Char 1 7c Child's guardian 6 AA17D Char 1 7d Other 7 AA18 Char 1 8 Plan to move in next 7 years 8 AA111 Char 1 11 Participate at another center 9 AA112 Char 1 12 Come to center every 4 months 10 AA113 Char 1 13 How often can come to center/year 11 AA114A Char 1 14a Car 12 AA114B Char 1 14b Taxi 13 AA114C Char 1 14c Bus/subway/metrolink 14 AA114D Char 1 14d Walked 15 AA114E Char 1 14e Other 16 AA115 Char 1 15 Trouble getting to center today 17 AA116 Char 1 16 Depend on someone for a ride 18 AA117 Char 1 17 Ever have transportation problems 19 AA118 Char 1 18 Any other children 20 AA119 Char 1 19 Trouble finding child care today 21 AA120 Char 1 20 Bring other children to center ever 22 AA121 Char 1 21 Bring other children how often 23 AA124 Char 1 24 Take work time to come to center 24 AA125 Char 1 25 Employer give time off for MD visit 25 AA126A Char 1 26a Take sick time 26 AA126B Char 1 26b Take vacation time 27 AA126C Char 1 26c Close business/office 28 AA126D Char 1 26d Make up time lost from work 29 AA126E Char 1 26e Other way to take time off from work 30 AA127 Char 1 27 Lose income when come to center 31 AA128 Char 1 28 Have a telephone during day 32 AA129 Char 1 29 Child has prescribed asthma med 33 AA130 Char 1 30 Takes medicine as prescribed 34 AA131 Char 1 31 Skips asthma medicine -

Mapping the Links Between ASEAN and the GMS, BIMP-EAGA, and IMT-GT
REGIONAL AND SUBREGIONAL PROGRAM LINKS Mapping the links between ASEAN and the GMS, BIMP-EAGA, and IMT-GT REGIONAL AND SUBREGIONAL PROGRAM LINKS Mapping the links between ASEAN and the GMS, BIMP-EAGA, and IMT-GT September 2013 © 2013 Asian Development Bank All rights reserved. Published 2013. Printed in the Philippines. ISBN 978-92-9254-202-3 (Print), 978-92-9254-203-0 (PDF) Publication Stock No. RPT135922-2 Cataloging-In-Publication Data Regional and Subregional Program Links: mapping the links between ASEAN and the GMS, BIMP-EAGA, and IMT-GT Mandaluyong City, Philippines: Asian Development Bank, 2013. 1. Regional cooperation and integration. 2. ASEAN. 3. GMS. 4. BIMP-EAGA. 5. IMT-GT. I. Asian Development Bank. The views expressed in this publication are those of the authors and do not necessarily reflect the views and policies of the Asian Development Bank (ADB) or its Board of Governors or the governments they represent. ADB does not guarantee the accuracy of the data included in this publication and accepts no responsibility for any consequence of their use. Use of the term “country” does not imply any judgment by the authors or ADB as to the legal or other status of any territorial entity. The symbol “$” represents the United States dollar unless otherwise indicated. “Asia” refers only to ADB’s Asian member economies. ADB encourages printing or copying information exclusively for personal and noncommercial use with proper acknowledgement of ADB. Users are restricted from reselling, redistributing, or creating derivative works for commercial purposes without the express, written consent of ADB. Photo credits (cover): ADB photo archive and Josephine Duque-Comia. -

Before the Public Service Commission of the State of Missouri
BEFORE THE PUBLIC SERVICE COMMISSION OF THE STATE OF MISSOURI In the Matter of Kansas City Power & Light ) Company’s Notice of Intent to File an ) Application for Authority to Establish a Demand- ) File No. EO-2015-0240 Side Programs Investment Mechanism ) In the Matter of KCP&L Greater Missouri Operations ) Company’s Notice of Intent to File an ) Application for Authority to Establish a Demand- ) File No. EO-2015-0241 Side Programs Investment Mechanism ) RESPONSE TO ORDER APPROVING APPLICATION TO MODIFY TECHNICAL RESOURCE MANUAL AND PROGRAM DESIGN INCENTIVE RANGES COMES NOW Kansas City Power & Light Company and KCP&L Greater Missouri Operations Company (the “Company”) and, in response Ordered Paragraph 2 of the Missouri Public Service Commission’s (“Commission”) March 21, 2018 Order Approving Application to Modify Technical Resource Manual and Program Design Incentive Ranges (“Order”), files the attached amended Appendix 1 and Appendix 2 (collectively referred to as “Amended Appendices”) to the Non-Unanimous Stipulation and Agreement (originally approved by the Commission on April 6, 2016). The Amended Appendices incorporate all modifications approved by the Commission in its Order. WHEREFORE, the Company respectfully requests the Commission consider this response to the Commission’s Order. Respectfully submitted, /s/ Roger W. Steiner Robert J. Hack MBN 36496 Roger W. Steiner MBN 39586 Kansas City Power & Light Company 1200 Main Street, 16th Floor Kansas City, MO 64105 (816) 556-2785 (Phone) (816) 556-2787 (Fax) [email protected] [email protected] James M. Fischer MBN 27543 Fischer & Dority, P.C. 101 Madison Street, Suite 400 Jefferson City, MO 65101 (573) 636-6758 (Phone) (573) 636-0383 (Fax) [email protected] Attorneys for Kansas City Power & Light Company and KCP&L Greater Missouri Operations Company CERTIFICATE OF SERVICE I do hereby certify that a true and correct copy of the foregoing document has been hand delivered, emailed or mailed, postage prepaid, this 26th day of March, 2018, to all counsel of record. -

Technical Assistance Layout with Instructions
Resettlement Plan August 2017 INO: Flood Management in Selected River Basins Sector Project Prepared by the Ministry of Public Works and Housing through the Directorate General of Water Resources for the Asian Development Bank. This is an updated and revised version of the draft originally posted in May 2015 available on http://www.adb.org/projects/35182-043/main#project- documents. CURRENCY EQUIVALENTS (as of 11 August 2016) Rp1.00 = $0.000076 $1.00 = Rp13,129 ABBREVIATIONS ADB – Asian Development Bank AH – affected household AP – affected person BAPPEDA – Badan Perencanaan Pembangunan Daerah (Provincial/District Development Planning Agency) BPN – Badan Pertanahan Nasional (National Land Agency) BWS – Balai Wilayah Sungai (Center for River Basin) CBFRM – community-based flood risk management COI – corridor of impact CWZ – construction works zone DED – detailed engineering design DGWR – Directorate General of Water Resources DMS – detailed measurement survey EA – executing agency EIA – environmental impact assessment EMA – External Monitoring Agency FMSRBSP – Flood Management in Selected River Basins Sector Project GOI – Government of Indonesia HH – household IA – Implementing agency IOL – inventory of losses IR – involuntary resettlement Km – kilometer LA – Land acquisition LAIT – land acquisition implementation team LRP – livelihood restoration program MAPPI – Masyarakat Profesi Penilai Indonesia (Indonesian Professional Appraiser Association) MOHA – Ministry of Home Affairs NJOP – Nilai Jual Object Pajal (tax object selling price) PIB -
Thailand Mega Project for GMS Connectivity the 9Th GMSARN International Conference 2014
Thailand Mega Project for GMS connectivity The 9th GMSARN International Conference 2014 Athibhu Chitranukroh Office of Transport and Traffic Policy and Planning Ministry of Transport, Thailand November 12th , 2014 Palace Hotel Saigon, Ho Chi Minh City, Vietnam Agenda : Thailand Mega Project for GMS connectivity Part 1: Trend of the future - Globalization & Global value chain - Urbanization - Critical Factor Part 2: Basic Fact of the GMS Part 3: Thailand - Logistics challenges - Infrastructure development program - Key projects Part 4 : Impact to the regional economy Trend : Globalization & Global Value Chain Effects of McDonalds and Starbuck’s franchises on global trade Source : The McGraw Center for teaching and learning Trend : Globalization & Global Value Chain : iPhone Trend : Globalization & Global Value Chain : Boeing 787 Trend : urbanization • 50% global GDP generated by 600 cities • Yr. 2025 : 40% global GDP will be generated by emerging markets Trend : urbanization Urban population • Yr. 1900 : 2 of 10 people live in urban • Yr. 2010 : 5 of 10 people live in urban • Yr. 2030 : 6 of 10 people live in urban • Yr. 2050 : 7 of 10 people live in urban Social : • Lack of jobs -> crime • Pollution -> disease • Traffic -> quality of life Environment : cities consume • 2/3 global energy • 60% water • CO2 70% Trend : urbanization Better Urbanization leads to higher-quality growth for all people - Urban & Transport infrastructure Trend : urbanization Trend : critical factor - Globalization & Global Value Chain - urbanization “Connectivity” -

Affordable Housing Supplementary Planning Document (2006)
London Borough of Hillingdon LOCAL DEVELOPMENT FRAMEWORK AFFORDABLE HOUSING SUPPLEMENTARY PLANNING DOCUMENT (2006) STATEMENTS OF CONSULTATION AND SUSTAINABILITY APPRAISAL Policy and Environmental Planning Team Planning and Transportation Group London Borough of Hillingdon MAY 2006 0 Statements of Consultation and Sustainability Appraisal CONTENTS 1.0 INTRODUCTION 2.0 CONSULTATION STATEMENT 2.1 Consultation arrangements 2.2 Comments received 3.0 SUSTAINABILITY APPRAISAL (SA) 3.1 Purpose of this statement and the questions it responds to 3.2 Integration of environmental considerations 3.3 Consultation 3.4 Reasons for choosing the adopted SPD 3.5 Monitoring 4.0 OUTCOME APPENDICES APPENDIX 1 – List of SPD and Sustainability Appraisal consultees APPENDIX 2 – Summary of representations and the council’s response Affordable Housing SPD London Borough of Hillingdon 1 Statements of Consultation and Sustainability Appraisal 1.0 INTRODUCTION 1.1 This document accompanies the adopted Hillingdon Affordable Housing SPD and it brings together the requirements under the Strategic Environmental Assessment (SEA) Directive (2001) and the Town and Country Planning Act 2004 for the preparation of both a Sustainability Appraisal Statement and a Consultation Statement at the adoption stage of any Supplementary Planning Document. 1.2 The Consultation Statement (Section 2 of this document) sets out the public participation and consultation process undertaken for the Affordable Housing SPD in accordance with regulation 18 (4) (b) of the Town and Country Planning (local Development) (England) Regulations 2004. 1.3 The preparation of the Sustainability Appraisal Statement (section 3 of this document) responds to the requirements of Article 9 of European Directive 2001/42/EC (Strategic Environmental Assessment (SEA) Directive), the Office of the Deputy Prime Minister’s ‘Sustainability Appraisal for Regional Spatial Strategies and Local Development Documents’ (2005) and ‘Environmental Assessment of Plans and Regulations’, (2004). -

Thesis Presented to the Graduate Faculty Of
A SEARCH FOR MULTI-DRUG RESISTANCE PUMP INHIBITOR MOLECULES BY ISOLATION OF NATURAL PRODUCTS Approved by: ________________________________ Edward R. Biehl, Professor ________________________________ John A. Maguire, Professor ________________________________ David Y. Son, Professor ________________________________ Pia D. Vogel, Professor A SEARCH FOR MULTI-DRUG RESISTANCE PUMP INHIBITOR MOLECULES BY ISOLATION OF NATURAL PRODUCTS A Thesis Presented to the Graduate Faculty of Dedman College Southern Methodist University In Partial Fulfillment of the Requirements For the degree of Master of Science With a Major in Chemistry By Alan Wilfred Humason (B.S., Chemistry, University of Massachusetts at Amherst) May 14, 2005 Humason, Alan B.S., University of Massachusetts at Amherst, 1979 A Search for Multi-Drug Resistance Pump Inhibitor Molecules By Isolation of Natural Products Advisor: Professor Edward R. Biehl Master of Science conferred May 14, 2005 Thesis completed April 22, 2005 A search for multi-drug resistant pump inhibitory molecules was conducted in several plants that have clinically, or in traditional medicine, demonstrated useful activity against bacteria. The investigation was conducted by extracting the plants with organic solvents, and fractionating the resultant extracts chromatographically. These fractions were then subjected to testing against Staphylococcus aureus, in conjunction with a sub-inhibitory dose of the antibiotic berberine. Extracts exhibiting inhibitory activity were further fractionated using vacuum liquid chromatography, flash liquid chromatography and conventional and preparative thin layer chromatography, attempting to isolate a single molecule which exhibits multi- drug resistance pump inhibition. The plants that were investigated, Eriogonum brevicaule Nuttall, Hydrastis canadensis, Thuja occidentalis, Rhus trilobata, Pouteria pallida, and Gunnera macrophylla, yielded over two hundred fractions, including several active ones. -

Transparency
Full schedule inside www.grapevine.is THE ESSENTIAL GUIDE TO LIFE, TRAVEL & ENTERTAINMENT IN ICELAND IN THE ISSUE Issue 2 • 2011 • February 4 - March 10 2011 + COMPLETE CITY LISTINGS - INSIDE! SHOPPING DAIRY #CABLEGATE MUSIC TRAVEL What will we do Skyr gets Siggi'd Greatest hits... The Sugarcubes: Snowboarding, without Havarí? did they world dominate glacier hiking: NICE! or die? Transparency WIKILEAKS IS NOT THE POINT! The info-wars have begun, and Iceland is begging to be the legislative battleground. Indeed, the so-called info-wars are raging, and Iceland is getting a piece of the action. Learn the story of Iceland's tryst with WikiLeaks and their love-child, the Icelandic Modern Media Initiative, whose plan to make the country into a transparency and information haven might put us back on the map. PAGE 10 The Reykjavík Grapevine Issue 2 — 2011 THE REYKJAVÍK GRAPEVINE Hafnarstræti 15, 101 Reykjavík 2 www.grapevine.is [email protected] Editorial | Haukur S. Magnússon Published by Fröken ehf. www.froken.is Member of the Icelandic Travel Industry Association SOME WWW.GRAPEVINE.IS EXCLUSIVES www.saf.is Haukur’s 36th Editorial Printed by Landsprent ehf. in 25.000 copies. ‘Cuz we can’t possibly fit all this awesome stuff on FUCK YOU, NEW YORK TIMES TRAVEL SECTION our pages EDITOR: Haukur S Magnússon / [email protected] But not really. More like: fuck all of us. -What is Crealism? JOURNALIST: Interview with Luis de Miranda, father of the ‘Creal- Anna Andersen / [email protected] A regular day at Grapevine HQ entails lots of trying devaluation of the krona that followed the country’s ism’ movement. -

A New Listing of Alberta Hunting, Resource Development and WISE
A New Listing of Alberta Hunting, Resource Development and WISE Foundation Revenue Stamps, and Stampless Licence, Authorization and Special Quota Licence Stamps and Cards 1964 to 1997 Compiled by Clayton Rubec and Dale Stover September 2013 Updated January 2014 Table of Contents Acknowledgements .............................................................................................................................. iii Preface ....................................................................................................................................................... iii Introduction .............................................................................................................................................. 1 Wildlife Branch and Departmental Names .................................................................................... 3 Observations ............................................................................................................................................. 3 Alberta Hunting Stamps: 1964 to 1997 ........................................................................................... 4 Alberta Resource Development Stamps: 1973 to 1997 .......................................................... 38 WISE Foundation Stamps: 1994 to 1997 ...................................................................................... 40 Special Licence Stamps: 1964 to 1997 .......................................................................................... 41 Special Stampless Licence