Report Prepared for the WPCS- 2015 a Preliminary Examination of the Genetic Variation Within and Between the Improvement Society Herds of Welsh Mountain Ponies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ring Time Type of Class Classes Judge 1 8.00 11.00 12.00 2.00 3.00
TIMETABLE (Classes will not start before the stated time) Ring Time Type of Class Classes Judge 1 8.00 Local Riders 101 – 103 Mr R Streets 11.00 Ladies Hunter (HOYS) 104 Jan Darwin 12.00 Side-Saddle 105 – 109 Mrs J Boardman 2.00 Novice Hunter 110 Jan Darwin - R Mr A Edmunds – C 3.00 Hacks 111 – 112 Jane Phillips Cobs 113 – 114 Jane Phillips Riding Horse 115 – 116 Jane Phillips 2 8.00 Show Hunter Ponies 201 – 208 Mrs Barfield 1.00 Show Ponies 209 - 215 Ms S L Bramwell 3 8.00 M & M Ridden (BSPS) 301 – 305 Mrs G Simpson M & M Ridden (NPS) 306 - 307 Mr R Sutcliffe 1.00 M & M In Hand 308 – 309 Mr R Sutcliffe 2.00 Palominos 310 – 312 Mrs S Hirons 3.00 Miniatures 313 - 315 Mrs L Hutley 4 8.00 Shetlands 401 – 407 Mrs J C Whitehouse 10.00 Welsh Section A & B 408 – 419 Mrs M H Hewitt 12.00 Welsh Section C & D 420 - 431 Mrs C Ingram Welsh Championship Supreme In Hand Championship (Lancashire Red Rose) – Mrs A Coles Whiteside & Knowles In Hand Championship (M&M) – Mrs A Coles 5 8.00 Working Hunter Ponies 501 – 507 Mrs J Griffin M & M Working Hunter 508 – 511 Mr R P Billington 1.00 Ponies Novice Working Hunter 512 - 514 Mr R P Billington 2.30 Ponies 6 8.00 Irish Draught Performance 601 Mrs J P Stacy - C class Miss V Smith - R 9.00 Novice Working Cob 602 Miss S Leatherbarrow 10.30 Working Hunter (HOYS) 603 Miss K Sears - R Mr A Edmunds - C 1.00 Novice Working Hunter 604 Miss K Sears - R Mrs D E Walton - C 7 8.00 Riding Pony Breeding 700 – 703 Mrs M E Mansfield 9.45 Hunter Pony Breeding 704 – 706 Mrs M E Mansfield 10.30 Private & Pleasure Driving 707 – 709 Mr J Baggaley 12.00 Hunter Breeding 710 – 714 Mrs K Swinnerton 2.30 IDHS 715 - 722 Mrs J P Stacy - C Miss V Smith -R 8 8.00 In-Hand Arabs 801 – 804 Mrs C Bennett 11.00 Ridden Arabs 805 – 806 Mrs C Bennett 2.30 Skewbald & Piebald 807 - 810 Mrs M E Mansfield 9 8.00 Coloured Horse and Pony 901 – 909 Mrs Linda Marsden Society 1.00 Veteran Horses 910 - 915 Mr D Vessey Main 8.00 Ridden Hunters M1 – M4 Mrs D E Walton - C 1 11.00 Mrs Ashby-Jones - R RING ONE LOCAL RIDERS Kindly sponsored by THE HULLAND SADDLERY Prizes in kind. -

1 2018 AGM Minutes V1.5.19 FINAL
1 The Welsh Pony and Cob Society Minutes of the Annual General Meeting of Members held at Venue Cymru, Llandudno 1 pm on Saturday 7 April 2018 Present The President, Mr Ifor Lloyd and 233 members who signed the attendance registers. In Attendance Sarah Case, MHA Broomfield Alexander and Doreen Walford, taking the minutes. The President welcomed everyone and declared the meeting open. The President noted that the meeting was also being audio-recorded and asked if there were any objections; there were none. He asked if anyone was making their own recording; there were none. He noted the Venue housekeeping matters. Members receiving awards were asked to remain at the close of the AGM for photographs. He asked if there were any objections to non-members attending, representing proxy votes or accepting awards. There were no objections. The President introduced Sarah Case from Broomfield Alexander who would declare the result of the ballot and Doreen Walford, taking shorthand minutes. Obituaries The President noted that a number of members had died during the year and extended the Society’s sympathy to families and friends. He called the meeting to stand for a moment’s silence. 1. Apologies Taken as read. 2. Minutes of the Annual General Meeting held on 17 April 2017 The President noted all the pages of the minutes individually and asked if there were any comments: there were none. The President called for adoption of the minutes. Seconded: Peter Rutherford. Carried by show of hands; no objections; no abstentions. Matters arising: None 3. Correspondence There was no correspondence. -

Irish Breeds Quiz Answers 1. What County In
Irish Breeds Quiz Answers 1. What county in Ireland does the Connemara Pony originate from? County Galway 2. Traditionally, for what purpose was the Irish Cob bred? To pull a cart / wagon To travel long distances To be suitable for children To be strong and sturdy 3. List three typical traits of the Irish Draught: 1. Versatile 2. Intelligent 3. Kind / willing nature Strong and sturdy, pulling / staying power / hardy 4. What are the two main composite breeds of the Irish Sport Horse? 1. Irish Draught 2. Thoroughbred 5. What is the smallest Irish native breed? Kerry Bog Pony 6. What three horse breeds can be included in a horse’s pedigree for it to qualify as a Traditional Irish Horse? Connemara Pony, Irish Draught, Thoroughbred 7. List three typical traits of the Connemara Pony: 1. Sure footed 2. Hardy 3. Calm / willing / kind temperament Staying power / intelligent / sound / athletic / versatile 8. Name one typical conformation trait of the Irish Cob: Stout, powerful, wide / short back, wide chest / good bone 9. For what activities were the Irish Draught originally bred? Farm work / pulling machinery, riding, hunting, driving 10. For what activities are Irish Draughts bred for today? Leisure / riding horses / allrounders, competition, cross breeding 11. What traits make the Irish Sport Horse so well suited to Equestrian sport today? Athleticism, jumping ability, courage, intelligence, soundness, kind temperament 12. What are the two main reasons for producing Kerry Bog Ponies? 1. To pull machinery 2. As riding ponies for children Companion ponies Showing . -

PIS the E-BARQ Questionnaire Will Take Approximately 20
05/10/2020 Qualtrics Survey Software English PIS The E-BARQ questionnaire will take approximately 20 - 30 minutes to complete. E-BARQ is voluntary and your information is confidential. If you answer all of the questions, you will receive a Share-&-Compare graph on completion. This graph will show you where your horse compares to the population on 14 different categories, including Trainability, Rideability, Social Confidence and so on. Please respond to all questions to receive your graph (which can be found on your E-BARQ dashboard (under the E-BARQ Results tab) , immediately on completion). Please click here to download the E-BARQ personal information statement. I have read and agreed to the Personal Information Statement and Terms and Conditions of the E-BARQ project. Yes No (this option will remove you from E-BARQ) https://sydney.qualtrics.com/Q/EditSection/Blocks/Ajax/GetSurveyPrintPreview?ContextSurveyID=SV_3dVyqziNawK514h&ContextLibraryID=U… 1/85 05/10/2020 Qualtrics Survey Software Your email address registered: ${e://Field/user} Is this your FIRST time completing an E-BARQ questionnaire? Select 'No' if you already have an E-BARQ Dashboard (have completed an E-BARQ for another horse). Yes No, I have completed an E-BARQ previously 1st E-BARQ Demographics Are you? In which country do you reside? https://sydney.qualtrics.com/Q/EditSection/Blocks/Ajax/GetSurveyPrintPreview?ContextSurveyID=SV_3dVyqziNawK514h&ContextLibraryID=U… 2/85 05/10/2020 Qualtrics Survey Software What is your age? Are you RIGHT or LEFT handed? Demographics Your horse's name: ${e://Field/horsename} Your horse's E-BARQ ID: ${e://Field/ebarqid} You are welcome to complete one E-BARQ for each horse that you own but this survey will refer only to the horse named here. -
Electronic Supplementary Material - Appendices
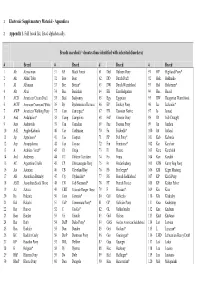
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

01622 633060 Kent Showground, Maidstone ME14
www.kentshow.co.uk 01622 633060 Kent Showground, Maidstone ME14 3JF KENT COUNTY AGRICULTURAL SOCIETY Patron: HRH The Duke of Kent, KG President: The Lord Colgrain, DL Chairman: Mr K Attwood OFFICIAL SCHEDULE OF THE EIGHTY NINTH KENT COUNTY SHOW FRIDAY - SATURDAY - SUNDAY 06 07 08 JULY 2018 ENTRIES CLOSE Cattle, Sheep, Wool and Goats - Friday 18 May 2018 Horses and Ponies - Friday 18 May 2018 Show Jumping - Friday 22 June 2018 SHOWGROUND HOLDING NUMBER 20/059/8000 Please send your entries to the Livestock and Equine Department Kent County Agricultural Society Kent Showground Detling, Maidstone Kent ME14 3JF Tel: 01622 630975 www.kentshowground.co.uk This Schedule is issued subject to the Rules, Orders and Regulations of the Department for the Environment, Food & Rural Affairs 1 PRESIDENTS AND DEPUTY PRESIDENTS PAST AND PRESENT 1923 The Right Honourable THE EARL OF DARNLEY, CA, JP, DL 1924-1925 R BRUCE WARD 1926 Capt J I H FRIEND, OBE, MC, JP, DL 1927 The Right Honourable LORD SACKVILLE, CBE, JP, DL 1928-1929 The Right Honourable THE EARL OF RADNOR, KG, KCVO 1930 The Right Honourable SIR PHILIP SASSOON, Bart, PC, MP 1931 Major G WHELER, MC 1932 Col The Right Honourable LORD CORNWALLIS CBE, JP, DL 1933 The Right Honourable LORD PLENDER, CBE, LLD, JP 1934-1935 R BRUCE WARD 1936-1937 W K WHIGHAM, JP 1938 The Right Honourable THE EARL OF RADNOR, KG, KCVO 1939 C TUFF, DL, JP, (Sir Charles Tuff) 1947-1981 The Right Honourable LORD CORNWALLIS, KCVO, KBE, MC 1947-1965 SIR EDWARD HARDY (Deputy President) 1966-1971 SIR LESLIE DOUBLEDAY JP (Deputy -

Dales Pony Society of America
Frutiger 45 Light - quarterly P Spotlight on thePalatino Breed Bold- Pony ITC Franklin Gothic As the Dales Pony Society celebrates its 100th anniversary in the UnitedP Kingdom, Pony Quarterly thought our readers might enjoy learning more about this rare and talented breed from across the Pond. THE ike many of the best pony breeds, the Dales Pony developed in the mountains and moors of Britain and Ireland. The nine British Native Pony breeds, romantically known as Mountain and Moorland or M&M ponies, are possessed of extraordinary strength. Fashioned by harsh and unforgiving environments, they are tough, sure-footed, with Lan inherent levelheadedness. While all of the M&M breeds, including the British Shetland, Connemara, Dartmoor, Exmoor,QUA Fell, Highland,RTER New Forest,LY and Welsh, are extraordinary animals, none are more PONYcommendable than the agile and talented Dales Pony. DALES PONY THE GREAT ALL-ROUNDER Story and photography by Kelly Davidson Dales Ponies were bred in the Yorkshire Dales of England in the 19th and early 20th centuries as pack ponies for the lead industry in the Pennine Mountains. As railways emerged and road systems improved, pack ponies were slowly phased out, but the Dales found a niche on the small farms in and around Yorkshire. With their unusual strength, and sensible natures, they offered great advantages to the small farmer and his family. In the early 1900’s, a Welsh Cob stallion named Comet was crossed to many high quality Dales mares, passing on a more free-moving shoulder and increased athleticism. The resulting stylish ponies, with their famous powerful and eye-catching trot, were unmatched in trotting races and provided a fashionable carriage drive into town for the family. -

32ND ANNUAL REGION III CONNEMARA SHOW July 28-29, 2018 – Wiley and Fletcher Arenas, Virginia Horse Center, Lexington, VA
32ND ANNUAL REGION III CONNEMARA SHOW July 28-29, 2018 – Wiley and Fletcher Arenas, Virginia Horse Center, Lexington, VA COMBINED TEST, DRESSAGE, JUMPERS, HUNTERS, TRAIL, FANCY DRESS AND GAMES - MOUNTAIN & MOORLAND DIVISION Please Join Us. Pre Enter by July 20, 2018 Friday Evening – Jan Redmond Handling/Trail Clinic 6 p.m. Saturday Exhibitors’ Dinner – free for all. ART SHOW – Bring your own art work for exhibit and sales or share your favorite equine art. Donna Duckworth and Alicia Daily are organizers – [email protected] or [email protected] 32nd Annual Region III Connemara Show at The Virginia Horse Center ~ Lexington, VA Saturday, July 28, and Sunday, July 29, 2018 DRESSAGE AND COMBINED TEST DRESSAGE RING: Saturday – 8 a.m. SHOW JUMPING: Saturday –9:30 a.m. Wiley Arena Dressage Judge: Tracey Smith-Oliver, Fairfield, VA Show Jumping Judge: Wayne Quarles, KY COMBINED TEST DRESSAGE DRESSAGE (SHOW JUMPING) D1 USDF 2015 Intro Level Test A CT1 USEF 2018 Training Eventing Test B — 3’3” D2 USDF 2015 Intro Level Test B CT2 USEF 2018 Novice Eventing Test B — 2’11” D3 USDF 2015 Intro Level Test C CT3 USEF 2018 Beg. Novice Eventing Test B — 2’7” D4 USEF 2018 Beg. Novice Eventing Test A CT4 USDF 2015 Intro Level Test B — up to 2’ D5 USEF 2018 Novice Eventing Test A D6 USEF 2018 Training Eventing Test A USEF RULES – EACH PONY MAY BE ENTERED IN D7 USEF Test of Choice Not Offered Above – Junior NO MORE THAN 3 DRESSAGE CLASSES AT THE and Senior (2 sets of ribbons for class; w/split determined SHOW. -

G2780 Horse Registries and Associations | University of Missouri Extension
G2780 Horse Registries and Associations | University of Missouri Extension http://extension.missouri.edu/publications/DisplayPrinterFriendlyPub.aspx?P=G2780 University of Missouri Extension G2780, Revised January 2006 Horse Registries and Associations Wayne Loch Department of Animal Sciences Light horses Albino International American Albino Association, Inc. (American Creme and American White Horse) Rt. 1, Box 20 Naper, Neb. 68755 Andalusian International Andalusian and Lusitano Horse Association 101 Carnoustie Box 115 Shoal Creek, Ala. 35242 205-995-8900 Fax 205-995-8966 www.andalusian.com Appaloosa Appaloosa Horse Club Inc. 5070 Hwy. 8 West Moscow, Idaho 83843 208-882-5578 Fax 208-882-8150 www.appaloosa.com 1 of 18 12/11/2009 4:16 PM G2780 Horse Registries and Associations | University of Missouri Extension http://extension.missouri.edu/publications/DisplayPrinterFriendlyPub.aspx?P=G2780 Arabian Arabian Horse Registry of America, Inc. PO Box 173886 Denver, Colo. 80217-3886 303-450-4748 Fax 303-450-2841 www.theregistry.org Inernational Arabian Horse Registry of North America and Partblood Arabian Registry of North America 12465 Brown-Moder Road. Marysville, Ohio 43040 Phone and Fax 937-644-5416 International Arabian Horse Association 10805 E. Bethany Dr. Aurora, Colo. 80014 303-696-4500 Fax 303-696-4599 iaha.com Missouri Arabian Horse Association 4340 Hwy. K New Haven, Mo. 63068 573-237-4705 American Bashkir Curly Registry Box 246 Ely, Nev. 89301 702-289-4999 Fax 702-289-8579 The Northwest Curly Horse Association 15521 216th Ave. NE Woodinville, Wash. 98072 206-788-9852 Buckskin American Buckskin Registry Association PO Box 3850 Redding, Calif. 96049-3850 Phone and Fax 916-223-1420 International Buckskin Horse Association 2 of 18 12/11/2009 4:16 PM G2780 Horse Registries and Associations | University of Missouri Extension http://extension.missouri.edu/publications/DisplayPrinterFriendlyPub.aspx?P=G2780 PO Box 357 St. -

Purification and Partial Characterization of a Coagulant Tle
Received: September 4, 2008 J Venom Anim Toxins incl Trop Dis. Accepted: February 13, 2009 V.15, n.3, p.411-423, 2009. Abstract published online: March 5, 2009 Original paper. Full paper published online: August 31, 2009 ISSN 1678-9199. PURIFICATION AND PARTIAL CHARACTERIZATION OF A COAGULANT SERINE PROTEASE FROM THE VENOM OF THE IRANIAN SNAKE Agkistrodon halys Ghorbanpur M (1), Zare Mirakabadi A (2), Zokaee F (1), Zolfagarrian H (2), Rabiei H (2) (1) Chemical Engineering Department, Amirkabir University, Tehran, Iran; (2) Department of Venomous Animals and Antivenom Production, Razi Vaccine and Serum Research Institute, Karaj, Iran. ABSTRACT: Agkistrodon halys is one of several dangerous snake species in Iran. Among the most important signs and symptoms in patients envenomated by this snake is disseminated intravascular coagulation. A thrombin-like enzyme, called AH143, was isolated from Agkistrodon halys venom by gel filtration on a Sephadex G-50 column, ion-exchange chromatography on a DEAE-Sepharose and high performance liquid chromatography (HPLC) on a C18 column. In the final stage of purification, 0.82 mg of purified enzyme was obtained from 182.5 mg of venom. The purified enzyme showed a single protein band by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), under reducing conditions, and its molecular mass was found to be about 30 kDa. AH143 revealed clotting activity in human plasma, which was not inhibited by EDTA or heparin. This enzyme still demonstrated coagulation activity when exposed to variations in temperature and pH ranging, respectively, from 30 to 40°C and from 7.0 to 8.0. -

9Th & 10Th Grade
1/27/2019 SpeechWire Tournament Services Ridge Invitational Declamation (9th & 10th Grade) Elimination round results Final round All Judges Code Name Team LK1 WK1 AA1 All ranks Place recips pref. AH101 Niamh Campbell Phoenix Country Day School 1 1 6 14 6.67 1 LP103 Matthew Floyd Catholic Memorial 2 2 1 15 5.25 2 BD109 Ashin Varghese Randolph High School 3 3 2 16 5.42 3 AO121 Juliette Gringeri Summit High School 5 4 4 23 3.95 4 UT102 Farrah Culmer Democracy Prep Harlem Prep 7 6 3 24 4.48 5 AH108 Enzo Acharya Phoenix Country Day School 6 5 7 26 4.01 6 AG101 Brynn Nelson Fontbonne Hall Academy 4 7 5 26 3.84 7 Semifinal round All Judges Code Name Team AP2 BD1 All ranks Place recips pref. AH101 Niamh Campbell Phoenix Country Day School 1 1 6 4.50 1 BD109 Ashin Varghese Randolph High School 8 4.25 2 UT102 Farrah Culmer Democracy Prep Harlem Prep 8 3.83 3 AH108 Enzo Acharya Phoenix Country Day School 8 3.50 4 AG101 Brynn Nelson Fontbonne Hall Academy 10 3.25 5 AO121 Juliette Gringeri Summit High School 10 3.25 5 LP103 Matthew Floyd Catholic Memorial 2 2 10 3.25 5 AG102 Juliann Bianco Fontbonne Hall Academy 11 3.53 8 AL101 Adrian Tejada Democracy Prep Endurance High 11 3.00 9 AH112 Milan Sewell Phoenix Country Day School 12 2.58 10 BC115 Maria Chabanov Xaverian HS 13 2.08 11 AH109 Devan Amin Phoenix Country Day School 14 2.37 12 GG105 Uswad Qureshi Phillipsburg HS 14 2.33 13 AC107 Nina Li Strath Haven High School 3 4 14 1.92 14 BC116 Amy Gruber Xaverian HS 15 2.70 15 AH133 Ella Brenes Phoenix Country Day School 4 5 15 2.28 16 AM101 Jaina Jallow Livingston High School 5 3 15 1.87 17 AK108 Fatimata Ly Achievement First 17 2.60 18 CZ112 Rajit Sharma Union Catholic 17 2.03 19 Semifinal round All Judges Code Name Team AA5 RY3 All ranks Place recips pref. -

Plan Local D'urbanisme
d ép a rt em AB15 e n ta le AB18 AB135 AB167 AB168 AB133 AB14 AB137 AB19 510 000 511 000 AB131 512 000 513 000 514 000 515 000 AB159 R AB27 u i AB20 464 000 465 000 s 466 000 467 000 468 000 469 000 AB134 s e A a AB21 n AB127 u ci en AB169 AB28 AB24 AB23 n°1 AB156 AB157 29 AB29 Ch AB130 AB138 AB158 s em er i AB22 av n Tr AB139 AB126 AB25 AB26 AB140 AB196 u AB142 d de AB145 A AB141 AB42 AB43 AB34 AB125 AB129 in N d m AB32 e he AB143 C Sim AB128 d or e AB35 re é Techen AB155 AB36 du AB124 AB61 u uissea R LA BORDENEUVE-OUEST AB144 AB44 AB1 AB189 AB37 AB60 à AB40 Ro ute AB147 AB11 AB64 N AB38 AB4 AB123 AB146 AB45 AB65 Sei ssa AB2 AB188 AB49 n AB46 AB50 AB10 AB9 AB39 AB59 AB151 AB62 AB63 AB122 Anc AB3 AB152 iednép CRABOTS-EST art eme AB187 S nta AB121 e le is sa AB66 n L Ro es ut te AB148 e ou AB58 AB12 AB5 é AB120 AB52 ch s AB164 em r in n 1 e 29 Av B179 AC118 a AB13 AB119 r Do AC119 T AB57 n-Gr abot AB149 Nag AB76 AB150 AB6 AB118 AB178 AC120 N AB67 CRABOTS-OUEST AC121 de AB17 de AB136 AB117 AB177 AB8 AB115 A Ruisseau AB160 AB56 AB68 AB16 AB165 AB132 S AC123 AB55 e 0 AB116 is d s a 0 ép à n a rt 0 em AB15 AB180 en 5 ta l 6 e AB18 AB54 AB192 AC125 AB114 u AC122 2 AB166 AB69 AB168 d a AB167 AB135 0 AB173 AB133 6 AB14 0 AB113 Ruis seau 0 S i AB112 AB194 m AB193 5 AB137 AB19 o Nh CAILLAOUAS-NORD r r 6 L AB70 'Is e le-en 2 AB109 AB175 -Dod AC117 don N AB131 on AB110 AB159 AC124 6 R AB27 u i AB20 s AB134 s e AB21 A AB181 à a nc AB162 R.