The Evolutionary Biology of Herbivorous Insects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

States Symbols State/ Union Territories Motto Song Animal / Aquatic
States Symbols State/ Animal / Foundation Butterfly / Motto Song Bird Fish Flower Fruit Tree Union territories Aquatic Animal day Reptile Maa Telugu Rose-ringed Snakehead Blackbuck Common Mango సతవ జయే Thalliki parakeet Murrel Neem Andhra Pradesh (Antilope jasmine (Mangifera indica) 1 November Satyameva Jayate (To Our Mother (Coracias (Channa (Azadirachta indica) cervicapra) (Jasminum officinale) (Truth alone triumphs) Telugu) benghalensis) striata) सयमेव जयते Mithun Hornbill Hollong ( Dipterocarpus Arunachal Pradesh (Rhynchostylis retusa) 20 February Satyameva Jayate (Bos frontalis) (Buceros bicornis) macrocarpus) (Truth alone triumphs) Satyameva O Mur Apunar Desh Indian rhinoceros White-winged duck Foxtail orchid Hollong (Dipterocarpus Assam सयमेव जयते 2 December Jayate (Truth alone triumphs) (O My Endearing Country) (Rhinoceros unicornis) (Asarcornis scutulata) (Rhynchostylis retusa) macrocarpus) Mere Bharat Ke House Sparrow Kachnar Mango Bihar Kanth Haar Gaur (Mithun) Peepal tree (Ficus religiosa) 22 March (Passer domesticus) (Phanera variegata) (Mangifera indica) (The Garland of My India) Arpa Pairi Ke Dhar Satyameva Wild buffalo Hill myna Rhynchostylis Chhattisgarh सयमेव जयते (The Streams of Arpa Sal (Shorea robusta) 1 November (Bubalus bubalis) (Gracula religiosa) gigantea Jayate (Truth alone triumphs) and Pairi) सव भाण पयतु मा किच Coconut palm Cocos दुःखमानुयात् Ruby Throated Grey mullet/Shevtto Jasmine nucifera (State heritage tree)/ Goa Sarve bhadrāṇi paśyantu mā Gaur (Bos gaurus) Yellow Bulbul in Konkani 30 May (Plumeria rubra) -

English Cop18 Prop. XXX CONVENTION ON
Original language: English CoP18 Prop. XXX CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal: To include the species Parides burchellanus in Appendix I, in accordance with Article II, paragraph 1 of the Convention and satisfying Criteria A i,ii, v; B i,iii, iv and C ii of Resolution Conf. 9.24 (Rev. CoP17). B. Proponent Brazil C. Supporting statement: 1. Taxonomy 1.1 Class: Insecta 1.2 Order: Lepidoptera 1.3 Family: Papilionidae 1.4 Species: Parides burchellanus (Westwood, 1872) 1.5 Synonymies: Papilio jaguarae Foetterle, 1902; Papilio numa Boisduval, 1836; Parides socama Schaus, 1902. 1.6 Common names: English: Swallowtail Portuguese:Borboleta-ribeirinha 2. Overview 1 The present proposal is based on the current knowledge about the species Parides burchellanus, well presented in Volume 7 of the Red Book of the Brazilian Fauna Threatened with Extinction1 and in present data on the supply of specimens for sale in the international market. The species has a restricted distribution2 with populations in the condition of decline as a consequence of anthropic actions in their habitat. It is categorized in Brazil as Critically Endangered (CR), according to criterion C2a(i) of the International Union for Conservation of Nature – IUCN. This criterion implies small and declining populations. In addition, these populations are also hundreds of kilometers apart from each other. The present proposal therefore seeks to reduce the pressure exerted by illegal trade on this species through its inclusion in Annex I to the Convention. -

Hot Spring Puddling by Butterflies
Ecologica Montenegrina 31: 46-49 (2020) This journal is available online at: www.biotaxa.org/em http://dx.doi.org/10.37828/em.2020.31.10 Hot spring puddling by butterflies YULIA S. KOLOSOVA*, OLGA V. AKSENOVA, ILYA V. VIKHREV & IVAN N. BOLOTOV N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences, Northern Dvina Emb. 23, 163000, Arkhangelsk, Russia *Corresponding author: [email protected] Received: 2 May 2020│ Accepted by V. Pešić: 20 May 2020 │ Published online: 23 May 2020. Puddling behavior of butterflies and moths is a well-known phenomenon driven by a deficit of several minerals and nutrients in larval and imago diet, especially sodium and proteins (Arms et al. 1974; Adler 1982; Boggs and Jackson 1991; Beck et al. 1999; John & Tennent 2012; Inoue et al. 2012). In particular, sodium and albumin were found to be the most attractive puddling resources for tropical butterflies on Borneo based on the results of a long-term experimental study (Beck et al. 1999). This kind of behavior is more characteristic for males, while female butterfly puddling occurs only occasionally (Beck et al. 1999; Adler & Pearson 1982; Scriber 1987, 2002; John & Tennent 2012; John & Dennis 2019). Male puddling could increase reproductive success in butterflies because minerals and nutrients are transferred through the spermatophore at mating (Boggs & Gilbert 1979; Pivnick & McNeil 1987; Smedley & Eisner 1996; Dennis et al. 2014; Mitra et al. 2016). It was shown that another purpose of puddling by males of swallowtail butterflies is to excrete excessive potassium (Inoue et al. -
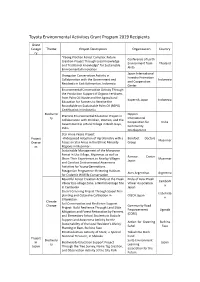
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

1996 No. 4 December
TROPICAL LEPIDOPTERA NEWS December 1996 No.4 LEPIDOPTERORUM CATALOGUS (New Series) The new world catalog of Lepidoptera renews the series title The new series (as edited by J. B. Heppner) began already in first begun in 1911. The original catalog series was published by 1989 with publication of the catalog of Noctuidae, by R. Poole. W. Junk Publishers of Berlin, Germany (later The Hague, E. J. Brill Publishers, of Leiden, Netherlands, published this first Netherlands), continuing until 1939 when the incomplete series fascicle in 3 volumes, covering already about a third of all known was deactivated due to World War II. The original series Lepidoptera. Since ATL took over the series, several families completed a large number of families between 1911 and 1939, have been readied for publication. Already this month, Fascicle totalling about 3 shelf-feet of text. Most Microlepidoptera, 48, on Epermeniidae, was published (authored by R. Gaedike, of however, were not covered, as also several macro families like the Deutsches Entomologisches Institut, Eberswalde, Germany). Noctuidae, and several families are incomplete (e.g., Geometridae In 1997, several other smaller families are expected, including and Pyralidae). Even for what was treated, the older catalogs are Acanthopteroctetidae (Davis), Acrolepiidae (Gaedike), Cecidosi now greatly out of date, due to the description of many new dae (Davis), Cercophanidae (Becker), Glyphipterigidae (Heppner), species and many changes in nomenclature over the last 5 to 8 Neotheoridae (Kristensen), Ochsenheimeriidae (Davis), Opostegi decades. dae (Davis), and Oxytenidae (Becker). Much of the publication The new series resembles the old series in some ways but it schedule depends on the cooperation of various specialists who will also have features not found in the old work. -

Sequestration of Aristolochic Acids from Meridic Diets by Larvae of Battus Polydamas Archidamas (Papilionidae: Troidini)
Eur. J. Entomol. 108: 41–45, 2011 http://www.eje.cz/scripts/viewabstract.php?abstract=1585 ISSN 1210-5759 (print), 1802-8829 (online) Sequestration of aristolochic acids from meridic diets by larvae of Battus polydamas archidamas (Papilionidae: Troidini) CARLOS F. PINTO1, ALEJANDRO URZÚA2 and HERMANN M. NIEMEYER1* 1Laboratorio de Química Ecológica, Universidad de Chile, Casilla 653, Santiago, Chile 2Laboratorio de Química Ecológica, Universidad de Santiago de Chile, Casilla 40, C-33 Santiago, Chile Key words. Lepidoptera, Papilionidae, Battus polydamas archidamas, Aristolochia chilensis, aristolochic acids, sequestration of toxins, uptake of toxins Abstract. Larvae of the butterfly, Battus polydamas archidamas (Papilionidae: Troidini) feed exclusively on aristolochic acid (AAs)-containing Aristolochia species (Aristolochiaceae). The distribution of sequestrated AAs in the tissues (body, integument and osmeterial secretions) of B. polydamas archidamas larvae during their development, when fed on a meridic diet containing either a higher or lower concentration of AAs (AAI and AAII) than occurs naturally in the aerial tissues of their host plant, was determined. Accumulation of AAs in the body and integument was proportional to the weight of larvae and greater in the larvae that fed on the diet containing the higher concentration of AAs. Phenolic AAs (AAIa and AAIVa) not present in the diets were found in all larval tissues examined. Integument and body extracts had a higher AAI/AAII ratio than in the original diet and also a relatively high AAIa/AAIVa ratio, suggesting a preferred AAII to AAIa transformation in those larval tissues. In the osmeterial secretion, the value of the AAI/AAII ratio was similar to that in the diets and the AAIa/AAIVa ratio close to 1, which suggests that hydroxylation of AAI to AAIVa and of AAII to AAIa occur to similar extents. -

Natural History of Fiji's Endemic Swallowtail Butterfly, Papilio Schmeltzi
32 TROP. LEPID. RES., 23(1): 32-38, 2013 CHANDRA ET AL.: Life history of Papilio schmeltzi NATURAL HISTORY OF FIJI’S ENDEMIC SWALLOWTAIL BUTTERFLY, PAPILIO SCHMELTZI (HERRICH-SCHAEFFER) Visheshni Chandra1, Uma R. Khurma1 and Takashi A. Inoue2 1School of Biological and Chemical Sciences, Faculty of Science, Technology and Environment, The University of the South Pacific, Private Bag, Suva, Fiji. Correspondance: [email protected]; 2Japanese National Institute of Agrobiological Sciences, Ôwashi 1-2, Tsukuba, Ibaraki, 305-8634, Japan Abstract - The wild population of Papilio schmeltzi (Herrich-Schaeffer) in the Fiji Islands is very small. Successful rearing methods should be established prior to any attempts to increase numbers of the natural population. Therefore, we studied the biology of this species. Papilio schmeltzi was reared on Micromelum minutum. Three generations were reared during the period from mid April 2008 to end of November 2008, and hence we estimate that in nature P. schmeltzi may have up to eight generations in a single year. Key words: Papilio schmeltzi, Micromelum minutum, life cycle, larval host plant, developmental duration, morphological characters, captive breeding INTRODUCTION MATERIALS AND METHODS Most of the Asia-Pacific swallowtail butterflies P. schmeltzi was reared in a screened enclosure from mid (Lepidopera: Papilionidae) belonging to the genus Papilio are April 2008 to end of November 2008. The enclosure was widely distributed in the tropics (e.g. Asia, Papua New Guinea, designed to provide conditions as close to its natural habitat as Australia, New Caledonia, Vanuatu, Solomon Islands, Fiji and possible and was located in an open area at the University of Samoa). -

Guidelines for the Capture and Management of Digital Zoological Names Information Francisco W
Guidelines for the Capture and Management of Digital Zoological Names Information Francisco W. Welter-Schultes Version 1.1 March 2013 Suggested citation: Welter-Schultes, F.W. (2012). Guidelines for the capture and management of digital zoological names information. Version 1.1 released on March 2013. Copenhagen: Global Biodiversity Information Facility, 126 pp, ISBN: 87-92020-44-5, accessible online at http://www.gbif.org/orc/?doc_id=2784. ISBN: 87-92020-44-5 (10 digits), 978-87-92020-44-4 (13 digits). Persistent URI: http://www.gbif.org/orc/?doc_id=2784. Language: English. Copyright © F. W. Welter-Schultes & Global Biodiversity Information Facility, 2012. Disclaimer: The information, ideas, and opinions presented in this publication are those of the author and do not represent those of GBIF. License: This document is licensed under Creative Commons Attribution 3.0. Document Control: Version Description Date of release Author(s) 0.1 First complete draft. January 2012 F. W. Welter- Schultes 0.2 Document re-structured to improve February 2012 F. W. Welter- usability. Available for public Schultes & A. review. González-Talaván 1.0 First public version of the June 2012 F. W. Welter- document. Schultes 1.1 Minor editions March 2013 F. W. Welter- Schultes Cover Credit: GBIF Secretariat, 2012. Image by Levi Szekeres (Romania), obtained by stock.xchng (http://www.sxc.hu/photo/1389360). March 2013 ii Guidelines for the management of digital zoological names information Version 1.1 Table of Contents How to use this book ......................................................................... 1 SECTION I 1. Introduction ................................................................................ 2 1.1. Identifiers and the role of Linnean names ......................................... 2 1.1.1 Identifiers .................................................................................. -

Ecological and Evolutionary Consequences of Variation in Aristolochic Acids, a Chemical Resource, for Sequestering Specialist Troidini Butterflies
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2013 Ecological and evolutionary consequences of variation in aristolochic acids, a chemical resource, for sequestering specialist Troidini butterflies Romina Daniela Dimarco University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Dimarco, Romina Daniela, "Ecological and evolutionary consequences of variation in aristolochic acids, a chemical resource, for sequestering specialist Troidini butterflies. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2567 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Romina Daniela Dimarco entitled "Ecological and evolutionary consequences of variation in aristolochic acids, a chemical resource, for sequestering specialist Troidini butterflies." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Ecology and Evolutionary Biology. James -

Japanese Papilio Butterflies Puddle Using Na Detected by Contact
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Naturwissenschaften (2012) 99:985–998 DOI 10.1007/s00114-012-0976-3 ORIGINAL PAPER Japanese Papilio butterflies puddle using Na+ detected by contact chemosensilla in the proboscis Takashi A. Inoue & Tamako Hata & Kiyoshi Asaoka & Tetsuo Ito & Kinuko Niihara & Hiroshi Hagiya & Fumio Yokohari Received: 11 July 2012 /Revised: 1 October 2012 /Accepted: 3 October 2012 /Published online: 9 November 2012 # The Author(s) 2012. This article is published with open access at Springerlink.com Abstract Many butterflies acquire nutrients from non- where the concentrations of K+,Ca2+, and/or Mg2+ were nectar sources such as puddles. To better understand how higher than that of Na+. This suggests that K+,Ca2+, and male Papilio butterflies identify suitable sites for puddling, Mg2+ do not interfere with the detection of Na+ by the Papilio we used behavioral and electrophysiological methods to butterfly. Using an electrophysiological method, tip record- examine the responses of Japanese Papilio butterflies to ings, receptor neurons in contact chemosensilla inside the Na+,K+,Ca2+, and Mg2+. Based on behavioral analyses, proboscis evoked regularly firing impulses to 1, 10, and + + 2+ these butterflies preferred a 10-mM Na solution to K ,Ca , 100 mM NaCl solutions but not to CaCl2 or MgCl2.The and Mg2+ solutions of the same concentration and among a dose–response patterns to the NaCl solutions were different tested range of 1 mM to 1 M NaCl. We also measured the ion among the neurons, which were classified into three types. -

Tera: Papilionidae): Cladistic Reappraisals Using Mainly Immature Stage Characters, with Focus on the Birdwings Ornithoptera Boisduval
Bull. Kitakyushu Mus. Nat. Hist., 15: 43-118. March 28, 1996 Gondwanan Evolution of the Troidine Swallowtails (Lepidop- tera: Papilionidae): Cladistic Reappraisals Using Mainly Immature Stage Characters, with Focus on the Birdwings Ornithoptera Boisduval Michael J. Parsons Entomology Section, Natural History Museum of Los Angeles County 900 Exposition Blvd., LosAngeles, California 90007, U.S.A.*' (Received December 13, 1995) Abstract In order to reappraise the interrelationships of genera in the tribe Troidini, and to test the resultant theory of troidine evolution against biogeographical data a cladistic analysis of troidine genera was performed. Data were obtained mainly from immature stages, providing characters that appeared to be more reliable than many "traditional" adult characters. A single cladogram hypothesising phylogenetic relation ships of the troidine genera was generated. This differs markedly from cladograms obtained in previous studies that used only adult characters. However, the cladogram appears to fit well biogeographical data for the Troidini in terms of vicariance biogcography, especially as this relates to the general hypotheses of Gondwanaland fragmentation and continental drift events advanced by recent geological studies. The genus Ornithoptera is shown to be distinct from Troides. Based on input data drawn equally from immature stages and adult characters, a single cladogram hypothesising the likely phylogeny of Ornithoptera species was generated. With minor weighting of a single important adult character (male -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt.