Tera: Papilionidae): Cladistic Reappraisals Using Mainly Immature Stage Characters, with Focus on the Birdwings Ornithoptera Boisduval
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

© CSIRO 2005 10.1071/IS04020 AC 1445-5226 Invertebrate Systematics, 2005, 19(2), 113–143
© CSIRO 2005 10.1071/IS04020_AC 1445-5226 Invertebrate Systematics, 2005, 19(2), 113–143. When and where did troidine butterflies (Lepidoptera : Papilionidae) evolve? Phylogenetic and biogeographic evidence suggests an origin in remnant Gondwana in the Late Cretaceous Michael F. BrabyA,B,D, John W. H. TruemanA and Rod EastwoodB,C ASchool of Botany and Zoology, The Australian National University, Canberra, ACT 0200, Australia. BMuseum of Comparative Zoology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA. CAustralian School of Environmental Studies, Griffith University, Nathan, Queensland 4111, Australia. DCorresponding author. Email: [email protected] The age, geographic origin and time of major radiation of the butterflies (Hesperioidea + Papilionoidea + Hedyloidea) are largely unknown. The general modern view is that butterflies arose during the Late Jurassic/Cretaceous in the southern hemisphere (southern Pangea/Gondwana before continental breakup), but this is not universally accepted, and is a best guess based largely on circumstantial evidence. The extreme paucity of fossils and lack of modern, robust, higher-level phylogenies of extant monophyletic groups have been major impediments towards determining reliable estimates of either their age or geographic origin. Here we present a phylogenetic and historical biogeographic analysis of a higher butterfly taxon, the swallowtail tribe Troidini. We analysed molecular data for three protein-encoding genes, mitochondrial ND5 and COI–COII, and nuclear EF–1α, both separately and in combination using maximum parsimony (with and without character weighting and transition/transversion weighting), maximum likelihood and Bayesian methods. Our sample included representatives of all 10 genera of Troidini and distant ingroup taxa (Baroniinae, Parnassiinae, Graphiini, Papilionini), with Pieridae as outgroup. -

MEET the BUTTERFLIES Identify the Butter Ies You've Seen at Butter Ies
MEET THE BUTTERFLIES Identify the butteries you’ve seen at Butteries LIVE! Learn the scientic, common name and country of origin. Experience the wonderful world of butteries with the help of Butteries LIVE! COMMON MORPHO Morpho peleides Family: Nymphalidae Range: Mexico to Colombia Wingspan: 5-8 in. (12.7 – 20.3 cm.) Fast Fact: Common morphos are attracted to fermenting fruits. WHITE MORPHO Morpho polyphemus Family: Nymphalidae Range: Mexico to Central America Wingspan: 4-4.75 in. (10-12 cm.) Fast Fact: Adult white morphos prefer to feed on rotting fruits or sap from trees. WHITENED BLUEWING Myscelia cyaniris Family: Nymphalidae Range: Mexico, parts of Central and South America Wingspan: 1.3-1.4 in. (3.3-3.6 cm.) Fast Fact: The underside of the whitened bluewing is silvery- gray, allowing it to blend in on bark and branches. MEXICAN BLUEWING Myscelia ethusa Family: Nymphalidae Range: Mexico, Central America, Colombia Wingspan: 2.5-3.0 in. (6.4-7.6 cm.) Fast Fact: Young caterpillars attach dung pellets and silk to a leaf vein to create a resting perch. NEW GUINEA BIRDWING Ornithoptera priamus Family: Papilionidae Range: Australia Wingspan: 5 in. (12.7 cm.) Fast Fact: New Guinea birdwings are sexually dimorphic. Females are much larger than the males, and their wings are black with white markings. LEARN MORE ABOUT SEXUAL DIMORPHISM IN BUTTERFLIES > MOCKER SWALLOWTAIL Papilio dardanus Family: Papilionidae Range: Africa Wingspan: 3.9-4.7 in. (10-12 cm.) Fast Fact: The male mocker swallowtail has a tail, while the female is tailless. LEARN MORE ABOUT SEXUALLY DIMORPHIC BUTTERFLIES > ORCHARD SWALLOWTAIL Papilio demodocus Family: Papilionidae Range: Africa and Arabia Wingspan: 4.5 in. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Alfred Russel Wallace and the Darwinian Species Concept
Gayana 73(2): Suplemento, 2009 ISSN 0717-652X ALFRED RUSSEL WALLACE AND THE Darwinian SPECIES CONCEPT: HIS paper ON THE swallowtail BUTTERFLIES (PAPILIONIDAE) OF 1865 ALFRED RUSSEL WALLACE Y EL concepto darwiniano DE ESPECIE: SU TRABAJO DE 1865 SOBRE MARIPOSAS papilio (PAPILIONIDAE) Jam ES MA LLET 1 Galton Laboratory, Department of Biology, University College London, 4 Stephenson Way, London UK, NW1 2HE E-mail: [email protected] Abstract Soon after his return from the Malay Archipelago, Alfred Russel Wallace published one of his most significant papers. The paper used butterflies of the family Papilionidae as a model system for testing evolutionary hypotheses, and included a revision of the Papilionidae of the region, as well as the description of some 20 new species. Wallace argued that the Papilionidae were the most advanced butterflies, against some of his colleagues such as Bates and Trimen who had claimed that the Nymphalidae were more advanced because of their possession of vestigial forelegs. In a very important section, Wallace laid out what is perhaps the clearest Darwinist definition of the differences between species, geographic subspecies, and local ‘varieties.’ He also discussed the relationship of these taxonomic categories to what is now termed ‘reproductive isolation.’ While accepting reproductive isolation as a cause of species, he rejected it as a definition. Instead, species were recognized as forms that overlap spatially and lack intermediates. However, this morphological distinctness argument breaks down for discrete polymorphisms, and Wallace clearly emphasised the conspecificity of non-mimetic males and female Batesian mimetic morphs in Papilio polytes, and also in P. -

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

June 2019 Number 191
June 2019 Number 191 In this issue... June Excursion..................................1 9 June - World Swallowtail Day.............................................................2 Orchard Swallowtail.............2 Ulysses Swallowtail.................3 Cairns Birdwing........................3 Amorphophallus - Camouflagued or just pretty? .......................................................................4 In flower this month......................5 What's Happening.........................6 The rare Megahertzia amplexicaulis in cultivation at Bayview Heights. Photo by Anthony Lagois via Facebook. Cairns Branch.............................6 Townsville Branch....................6 Tablelands Branch...................6 June Excursion June's excursion will take us to the private garden of Anthony Lagois and Brian Moran. Situated on the Cairns hillslopes in Bayview Heights, the garden contains a unique and expanding collection of native rainforest plants. Many things grown here are rarely seen in cultivation. This month's excursion will commence a little earlier than usual - 10 a.m. See the last page for directions and parking instructions. Page 1 SGAP Cairns Branch - Newsletter 191 9 June - World Swallowtail Day The British "Swallowtail and Birdwing Butterfly Trust" have declared 9 June 2019 to be the World Swallowtail Day. This British conservation initiative provides an opportunity to discuss some of our native swallowtail butterflies, and the native plants they eat. Britain's swallowtail butterfly, Papilio machaon is the island nation's -
List of Previous Grant Projects
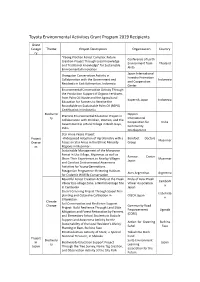
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

For Enumeration of This Part a Linear Sequence of Lycophytes and Ferns After Christenhusz, M
PTERIDOPHYTA For enumeration of this part A linear sequence of Lycophytes and Ferns after Christenhusz, M. J. M.; Zhang, X.C. & Schneider, H. (2011) has been followed Subclass: Lycopodiidae Beketov (1863). Order: Selaginellales (1874). Selaginellaceae Willkomm, Anleit. Stud. Bot. 2: 163. 1854; Prodr. FI. Hisp. 1(1): 14. 1861. SELAGINELLA P. Beauvois, Megasin Encycl. 9: 478. 1804. Selaginella monospora Spring, Mém. Acad. Roy. Sci. Belgique 24: 135. 1850; Monogr. Lyc. II:135. 1850; Alston, Bull. Fan. Mem. Inst. Biol. Bot. 5: 288, 1954; Alston, Proc. Nat. Inst. Sc. Ind. 11: 228. 1945; Reed, C.F., Ind. Sellaginellarum 160 – 161. 1966; Panigrahi et Dixit, Proc. Nat. Inst. Sc. Ind. 34B (4): 201, f.6. 1968; Kunio Iwatsuki in Hara, Fl. East. Himal. 3: 168. 1972; Ghosh et al., Pter. Fl. East. Ind. 1: 127. 2004. Selaginella gorvalensis Spring, Monogr. Lyc. II: 256. 1850; Bak, Handb. Fern Allies 107. 1887; Selaginella microclada Bak, Jour. Bot. 22: 246. 1884; Selaginella plumose var. monospora (Spring) Bak, Jour. Bot. 21:145. 1883; Selaginella semicordata sensu Burkill, Rec. Bot. Surv. Ind. 10: 228. 1925, non Spring. Plant up to 90 cm, main stem prostrate, rooting on all sides and at intervals, unequally tetragonal, main stem alternately branched 5 – 9 times, branching unequal, flexuous; leavesobscurely green, dimorphus, lateral leaves oblong to ovate-lanceolate, subacute, denticulate to serrulate at base. Spike short, quadrangular, sporophylls dimorphic, large sporophyls less than half as long as lateral leaves, oblong- lanceolate, obtuse, denticulate, small sporophylls dentate, ovate, acuminate. Fertile: October to January. Specimen Cited: Park, Rajib & AP Das 0521, dated 23. 07. -

Mise En Page 1
RIEUNIER - M UIZON HISTOIRE NATURELLE Lundi 9 Novembre 2015 Paris - Drouot 1 - 6 132 RIEUNIER - M UIZON Olivier Rieunier et Vincent de Muizon Commissaires-Priseurs Associés VENTE AUX ENCHÈRES PUBLIQUES : LUNDI 9 NOVEMBRE 2015 HÔTEL DROUOT - SALLE 10 9, rue Drouot - 75009 Paris HISTOIRE NATURELLE à 14H ENTOMOLOGIE Bel ensemble d’Insectes exotiques : Lépidoptères - Coléoptères- Orthoptères… ORNITHOLOGIE spécimens issus d’élevage ainsi que d’une très ancienne collection COQUILLAGES ET DIVERS OUVRAGES D’HISTOIRE NATURELLE Expert : Gilbert LACHAUME Entomologiste, Expert en Histoire Naturelle 4, rue Duméril - 75013 Paris - Tél./Fax : +33 11 48 77 61 20 - [email protected] EXPOSITIONS PUBLIQUES : DROUOT - SALLE 10 SAMEDI 7 NOVEMBRE 2015 DE 11H À 18H LUNDI 9 NOVEMBRE 2015 DE 11H À 12H Téléphone pendant les expositions et la vente : 01 48 00 20 10 Une grande partie des lots est reproduite sur notre site : www.rieunierassocies.com RIEUNIER - de MUIZON SARL 10, rue Rossini – 75009 Paris - Tél : +33 1 47 70 32 32 - Télécopie : +33 1 47 70 32 33 E-mail : [email protected] - Site internet : www.rieunier-associes.com OVV N° Agrément 2002-293 du 27-06-02 Maître Olivier Rieunier Commissaire - Priseur judiciaire ORNITHOLOGIE- CONCHYLIOLOGIE et divers Tous les d’oiseaux correspondants aux numéros 1 à 81 inc. sont nés et ont été élevés en captivité dans l’ U.E. à l'exception de quelques espèces dont la chasse est autorisée. Les spécimens appartenant à des espèces inscrites en Annexe A (du J.O. de l'U.E.) sont accompagnés du C.I.C. réglementaire. Les spécimens appartenant à des espèces inscrites en Annexe B ou C (du J.O. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

The Evolutionary Biology of Herbivorous Insects
GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 1 Aptara (PPG-Quark) PART I EVOLUTION OF POPULATIONS AND SPECIES GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 2 Aptara (PPG-Quark) GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 3 Aptara (PPG-Quark) ONE Chemical Mediation of Host-Plant Specialization: The Papilionid Paradigm MAY R. BERENBAUM AND PAUL P. FEENY Understanding the physiological and behavioral mecha- chemistry throughout the life cycle are central to these nisms underlying host-plant specialization in holo- debates. Almost 60 years ago, Dethier (1948) suggested that metabolous species, which undergo complete development “the first barrier to be overcome in the insect-plant relation- with a pupal stage, presents a particular challenge in that ship is a behavioral one. The insect must sense and discrim- the process of host-plant selection is generally carried out inate before nutritional and toxic factors become opera- by the adult stage, whereas host-plant utilization is more tive.” Thus, Dethier argued for the primacy of adult [AQ2] the province of the larval stage (Thompson 1988a, 1988b). preference, or detection and response to kairomonal cues, Thus, within a species, critical chemical, physical, or visual in host-plant shifts. In contrast, Ehrlich and Raven (1964) cues for host-plant identification may differ over the course reasoned that “after the restriction of certain groups of of the life cycle. An organizing principle for the study of insects to a narrow range of food plants, the formerly repel- host-range evolution is the preference-performance hypoth- lent substances of these plants might . -

Chapter 6 ENUMERATION
Chapter 6 ENUMERATION . ENUMERATION The spermatophytic plants with their accepted names as per The Plant List [http://www.theplantlist.org/ ], through proper taxonomic treatments of recorded species and infra-specific taxa, collected from Gorumara National Park has been arranged in compliance with the presently accepted APG-III (Chase & Reveal, 2009) system of classification. Further, for better convenience the presentation of each species in the enumeration the genera and species under the families are arranged in alphabetical order. In case of Gymnosperms, four families with their genera and species also arranged in alphabetical order. The following sequence of enumeration is taken into consideration while enumerating each identified plants. (a) Accepted name, (b) Basionym if any, (c) Synonyms if any, (d) Homonym if any, (e) Vernacular name if any, (f) Description, (g) Flowering and fruiting periods, (h) Specimen cited, (i) Local distribution, and (j) General distribution. Each individual taxon is being treated here with the protologue at first along with the author citation and then referring the available important references for overall and/or adjacent floras and taxonomic treatments. Mentioned below is the list of important books, selected scientific journals, papers, newsletters and periodicals those have been referred during the citation of references. Chronicles of literature of reference: Names of the important books referred: Beng. Pl. : Bengal Plants En. Fl .Pl. Nepal : An Enumeration of the Flowering Plants of Nepal Fasc.Fl.India : Fascicles of Flora of India Fl.Brit.India : The Flora of British India Fl.Bhutan : Flora of Bhutan Fl.E.Him. : Flora of Eastern Himalaya Fl.India : Flora of India Fl Indi.