Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endemic Chromoblastomycosis Caused Predominantly by Fonsecaea Nubica, Madagascar
Article DOI: https://doi.org/10.3201/eid2606.191498 Endemic Chromoblastomycosis Caused Predominantly by Fonsecaea nubica, Madagascar Appendix Appendix Table. Characteristics of clinical isolates from patients with suspected chromoblastomycosis in Madagascar and reference strains used in this study* Panfugal PCR for S/C Specific PCR for S/C MALDI-ToF GenBank accession no. Ccar- Code Species for D1D2, ITS Source Origin NL-1/NL-4 ITS1/ITS4 F/Ccar-R Fon-F/Fon-R MSP ID MYC04002 Unidentified NA, NA Biopsy Vatovavy Fitovinany N/P P/N N/N N/NA NA NA MYC04004 Fonsecaea sp. NA, NA Biopsy Vatovavy Fitovinany P/P P/NA N/NA N/P NA NA MYC08006 Fonsecaea sp. MK828325, NA Biopsy Analanjirofo P/P P/NA N/N N/P NA NA MYC10010 Fonsecaea sp. NA, NA Biopsy Melaky P/P P/NA N/NA P/P NA NA MYC10011 Fonsecaea sp. NA, NA Squama Vatovavy Fitovinany P/P P/NA N/NA P/P NA NA MYC10014 Cladophialophora carrionii MK828362, MK820046 Squama Androy P/P P/P N/P N/N X NA MYC03022 Unidentified NA, NA Biopsy Sofia NA/NA NA/NA N/NA NA/NA NA NA MYC04026 Fonsecaea nubica MK828326, MK828125 Biopsy Alaotra Mangoro N/P N/P NA/N NA/P NA X MYC05032 F. nubica MK828327, MK828124 Squama Amoron I Mania N/P N/P NA/N NA/P NA NA MYC06033 Unidentified NA, NA Biopsy Androy N/P N/P NA/N NA/N NA NA MYC08040 C. carrionii MK828363, MK820047 Biopsy Atsimo Andrefana N/P N/P NA/P NA/N NA NA MYC09041 Unidentified NA, NA Biopsy Analamanga NA/NA NA/NA NA/NA NA NA NA MYC03048 Unidentified NA, NA Biopsy Vakinankaratra N/P N/N N/N N/N NA NA MYC06057 Unidentified NA, NA Biopsy Amoron I Mania P/P N/N N/N N/N NA NA MYC06059 F. -

Rep 2 out Public 2010 S Tlet Sur of Ma Urvey Rvey Adagas Repor Scar Rt
Evidence for Malaria Medicines Policy Outlet Survey Republic of Madagascar 2010 Survey Report MINSTERE DE LA SANTE PUBLIQUE www. ACTwatch.info Copyright © 2010 Population Services International (PSI). All rights reserved. Acknowledgements ACTwatch is funded by the Bill and Melinda Gates Foundation. This study was implemented by Population Services International (PSI). ACTwatch’s Advisory Committee: Mr. Suprotik Basu Advisor to the UN Secretary General's Special Envoy for Malaria Mr. Rik Bosman Supply Chain Expert, Former Senior Vice President, Unilever Ms. Renia Coghlan Global Access Associate Director, Medicines for Malaria Venture (MMV) Dr. Thom Eisele Assistant Professor, Tulane University Mr. Louis Da Gama Malaria Advocacy & Communications Director, Global Health Advocates Dr. Paul Lavani Executive Director, RaPID Pharmacovigilance Program Dr. Ramanan Senior Fellow, Resources for the Future Dr. Matthew Lynch Project Director, VOICES, Johns Hopkins University Centre for Dr. Bernard Nahlen Deputy Coordinator, President's Malaria Initiative (PMI) Dr. Jayesh M. Pandit Head, Pharmacovigilance Department, Pharmacy and Poisons Board‐Kenya Dr. Melanie Renshaw Advisor to the UN Secretary General's Special Envoy for Malaria Mr. Oliver Sabot Vice‐President, Vaccines Clinton Foundation Ms. Rima Shretta Senior Program Associate, Strengthening Pharmaceutical Systems Dr. Rick Steketee Science Director, Malaria Control and Evaluation Partnership in Africa Dr. Warren Stevens Health Economist Dr. Gladys Tetteh CDC Resident Advisor, President’s Malaria -

RAPPORT D'activité 2015-2016 Projet D'adaptation De La Gestion Des Zones Côtières Au Changement Climatique
17' 0( (/ 1( ¶( 1 & 2 2 5 / , 2 9 * 1 , ( ( ¶ / ( 7 ( ' ' ( ( 6 5 ) ( 2 7 6 5 , ( 1 , 7 6 0 MINISTERE DE L’ENVIRONNEMENT, DE L’ECOLOGIE ET DES FORETS SECRETARIAT GENERAL BUREAU NATIONAL DE COORDINATION DES CHANGEMENTS CLIMATIQUES RAPPORT D'ACTIVITÉ 2015-2016 Projet d'Adaptation de la gestion des zones côtières au changement climatique PROJET D’AdaptatioN DE LA GESTION DES ZONES CÔTIÈRES AU CHANGEMENT CLIMatiQUE Etant un pays insulaire, Madagascar est Plusieurs actions ont été entreprises par le considéré comme l’un des pays les plus projet d’Adaptation de la gestion des Zones SOMMAIRE vulnérables à la variabilité et aux changements Côtières au changement climatique en tenant climatiques. Les dits changements se compte de l’Amélioration des écosystèmes CONTEXTE 5 manifestent surtout par le «chamboulement et des moyens de subsistance » au cours du régime des pluviométries, l’augmentation de l’année 2016 comme la réalisation des COMPOSANTE 1 : RENForcement DES capacITÉS de la température, la montée du niveau de études de vulnérabilité dans les quatre zones INSTITUTIONNELLES AUX Impacts DU CHANGEMENT la mer et l’intensification des évènements d’intervention, la création d’un mécanisme de CLImatIQUE DANS LES SITES DU proJET climatiques extrêmes tels que les cyclones, les coordination et la mise en place de la Gestion (MENABE, BOENY, VatovavY FItovINANY ET ATSINANANA) 7 inondations et les sècheresses. Devant cette Intégrée des zones côtières dans les régions situation alarmante, des actions d’adaptation Atsinanana, Boeny, et Vatovavy Fitovinany, ainsi COMPOSANTE 2 : RÉHABILItatION ET GESTION DES ZONES sont déja mises en oeuvre à Madagascar afin de que la mise en œuvre des scénarios climatiques CÔTIÈRES EN VUE d’uNE RÉSILIENCE À LONG TERME 17 renforcer la résilience de la population locale et à l’échelle réduite de ces quatre régions. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Universite D'antananarivo
UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE PHYSIQUE MEMOIRE Pour l’obtention du diplôme de MAITRISE DES SCIENCES ET TECHNIQUES EN GEOPHYSIQUE APPLIQUEE Option : Mines et Environnement Intitulé ETUDE DES ZONES FAVORABLES EN MINERALISATION AURIFERE ET EN EMERAUDE DANS LA COMMUNE RURALE D’ANDRORANGAVOLA, DISTRICT D’IFANADIANA, REGION VATOVAVY FITOVINANY Présenté par RANTOSOA Andriharizafy Devant la commission d’examen composée de : Président : RANDRIAMANANTANY Zely Arivelo Professeur Titulaire Rapporteur : RASOLOMANANA Eddy Professeur Examinateurs: RANDRIANJA Roger Professeur RAZAFINDRAKOTO Boni Gauthier Docteur Le 29 Décembre 2008 UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE PHYSIQUE MEMOIRE Pour l’obtention du diplôme de MAITRISE DES SCIENCES ET TECHNIQUES EN GEOPHYSIQUE APPLIQUEE Option : Mines et Environnement Intitulé ETUDE DES ZONES FAVORABLES EN MINERALISATION AURIFERE ET EN EMERAUDE DANS LA COMMUNE RURALE D’ANDRORANGAVOLA, DISTRICT D’IFANADIANA, REGION VATOVAVY FITOVINANY Présenté par RANTOSOA Andriharizafy Devant la commission d’examen composée de : Président : RANDRIAMANANTANY Zely Arivelo Professeur Titulaire Rapporteur : RASOLOMANANA Eddy Professeur Examinateurs: RANDRIANJA Roger Professeur RAZAFINDRAKOTO Boni Gauthier Docteur Le 29 Décembre 2008 REMERCIEMENTS En préambule de ce mémoire, je souhaite adresser ici mes vifs remerciements à toutes personnes qui, de près ou de loin, ont contribué à l'élaboration de ce mémoire tout particulièrement les personnes citées ci après : Madame RANDRIAMANANTANY Zely Arivelo , Professeur Titulaire, Chef du Département de Physique, qui a bien voulu présidé le membre de jury. Monsieur le Professeur RANAIVO Nomenjanahary Flavien , Directeur de l’ Institut et Observatoire de Géophysique d’Antananarivo (IOGA), Responsable Pédagogique de la formation en Maîtrise des Science et Technique en Géophysique Appliqué (MSTGA), et qui m’a accepté d’être parmi ses étudiants au sein dudit établissement. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
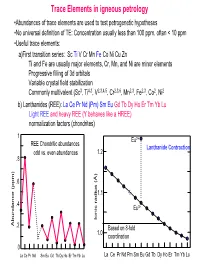
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand?
The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand? Shun-ichiro Karato, David Bercovici, Garrett Leahy, Guillaume Richard and Zhicheng Jing Yale University, Department of Geology and Geophysics, New Haven, CT 06520 Materials circulation in Earth’s mantle will be modified if partial melting occurs in the transition zone. Melting in the transition zone is plausible if a significant amount of incompatible components is present in Earth’s mantle. We review the experimental data on melting and melt density and conclude that melting is likely under a broad range of conditions, although conditions for dense melt are more limited. Current geochemical models of Earth suggest the presence of relatively dense incompatible components such as K2O and we conclude that a dense melt is likely formed when the fraction of water is small. Models have been developed to understand the structure of a melt layer and the circulation of melt and vola- tiles. The model suggests a relatively thin melt-rich layer that can be entrained by downwelling current to maintain “steady-state” structure. If deep mantle melting occurs with a small melt fraction, highly incompatible elements including hydro- gen, helium and argon are sequestered without much effect on more compatible elements. This provides a natural explanation for many paradoxes including (i) the apparent discrepancy between whole mantle convection suggested from geophysical observations and the presence of long-lived large reservoirs suggested by geochemical observations, (ii) the helium/heat flow paradox and (iii) the argon paradox. Geophysical observations are reviewed including electrical conductivity and anomalies in seismic wave velocities to test the model and some future direc- tions to refine the model are discussed. -

Le Developpement Economique De La Region Vatovavy Fitovinany
UNIVERSITE D’ANTANANARIVO Année Universitaire : 2006-2007 Faculté de Droit, d’Economie, de Second Cycle – Promotion Sortante Gestion et de Sociologie Option : DEVELOPPEMENT DEPARTEMENT ECONOMIE « Promotion ANDRAINA » Mémoire de fin de Cycle LE DEVELOPPEMENT ECONOMIQUE DE LA REGION VATOVAVY FITOVINANY Encadré par : Monsieur Gédéon RAJAONSON Présenté par : MANIRISOA RAZAFIMARINTSARA Firmin Date de soutenance : 14 Décembre 2007 REMERCIEMENTS Pour commencer, je tiens à exprimer toute ma reconnaissance à tous ceux qui ont contribué, de près ou de loin, à ma formation et à la réalisation de ce Grand Mémoire de fin d’études en Economie. J’adresse donc tout particulièrement mes vifs remerciement à : • DIEU TOUT PUISSANT • Mon encadreur Monsieur Gédéon RAJAONSON ; • Tous les enseignants et les Personnels administratifs du Département Economie de la Faculté DEGS de l’Université d’Antananarivo ; • Monsieur Le Chef de Région de Vatovavy Fitovinany et ses équipes • Monsieur le Directeur Régional des Travaux Publics de Vatovavy Fitovinany • Ma famille pour leurs soutiens permanents. Veuillez accepter le témoignage de ma profonde gratitude. LISTE DES ABREVIATIONS ANGAP : Agence Nationale de la Gestion des Aires Protégés CEG : Collège d’Enseignement Général CHD 1 : Centre Hospitalier de District Niveau 1 CHD 2 : Centre Hospitalier de District Niveau 2 CISCO : Circonscription Scolaire CSB 1 : Centre de Santé de Base Niveau 1 CSB 2 : Centre de Santé de Base Niveau 2 DRDR : Direction Régionale du Développement Rural EPP : Ecole Primaire Public FCE : Fianarantsoa Côte Est FER : Fonds d’Entretien Routier FTM : Foibe Toantsritanin’i Madagasikara GU : Guichet Unique HIMO : Haute Intensité de Main d’œuvre INSTAT : Institut National de la Statistique M.A.E.P. -

Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer 2. 1Department Of
47th Lunar! and Planetary Science Conference (2016) 1058.pdf Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer2. 1Department of Earth and Environmental Science, New Mexico Tech, Socorro, New Mexico 87801, 2 Institute of Meteoritics, Department of Earth and Planetary Science, University of New Mexico, Albuquerque, New Mexico 87131, 2 NASA Johnson Space Center, Houston, TX 77058. Introduction: Because ratios of elements with group centered near Earth’s primitive mantle (PM) similar incompatibilities do not change much with composition (Fig. 2). This distribution is similar to degree of melting or fractional crystallization in the the distribution of most mare basalts from the Moon mantles of silicate planets, they are useful in sampled by the Apollo missions, except for the high- characterizing compositions of mantle sources of Ti basalts from Apollo 11 and 17 both of which are basalts that have not interacted with continental crust enriched in Nb. The primitive mantle grouping is [1,2]. Using Nb/Th and Zr/Nb ratios in young especially evident for Apollo 11, 12 and 15 basalts in oceanic basalts, three mantle domains can be which samples cluster tightly around primitive identified [3]: enriched (EM), depleted (DM), and mantle (PM) composition on Zr/Nb-Nb/Th, Th/Yb- hydrated mantle (HM) (Fig. 1). DM is characterized Nb/Yb and La/Sm-Nb/Th diagrams (Fig. 3). Very by high values of both ratios (Nb/Th > 8; Zr/Nb > 20) low-Ti green volcanic glasses (and some due to Th depletion relative to Nb, and to Nb intermediate-Ti yellow volcanic glasses) plot in a depletion relative to Zr. -

Durham Research Online
Durham Research Online Deposited in DRO: 14 March 2018 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Namur, Olivier and Humphreys, Madeleine C.S. (2018) 'Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland.', Journal of petrology., 59 (3). pp. 387-418. Further information on publisher's website: https://doi.org/10.1093/petrology/egy032 Publisher's copyright statement: This is a pre-copyedited, author-produced version of an article accepted for publication in Journal Of Petrology following peer review. The version of record Namur, Olivier Humphreys, Madeleine C.S. (2018). Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland. Journal of Petrology is available online at: https://doi.org/10.1093/petrology/egy032. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Trace element constraints on the differentiation and crystal mush solidification in the Skaergaard intrusion, Greenland Olivier Namur1*, Madeleine C.S.