Mineral Potential for Incompatible Element Deposits Hosted In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Breakthrough Chemistry Simulations for Lithium Processing Contents
think simulation | getting the chemistry right Breakthrough chemistry simulations for lithium processing Using simulation to maximize your investment return in lithium extraction and processing Contents Introduction ............................................................................................................................................ 2 Process simulation deficiencies ................................................................................................................ 2 OLI Systems electrolyte thermodynamics .................................................................................................2 OLI Systems lithium initiative .................................................................................................................... 2 Lithium phase 1 and potash chemistry is complete ................................................................................... 2 Fundamental sulfate – chloride systems .............................................................................................. 3 Fundamental hydroxide and carbonate systems .................................................................................. 3 Lithium in acid environments for processing and recycling ................................................................... 3 Lithium borate systems for Li production ............................................................................................. 4 Systems related to Li hydrometallurgical processing, purifications and recycling .................................. -

5. the Fossil Fuels: Petroleum I - Finding Oil
5. The Fossil Fuels: Petroleum I - Finding Oil 5.1 Introduction Finding petroleum is a difficult and sometimes dangerous job. It involves a large number of individuals with a wide variety of skills. These include geologists, seismologists, various types of engineers and landmen. All of these individuals play vital but specific roles in the development and ultimate production of a play. This lab conveys just a portion of the complexities involved in this important search. Figure 1: Drill pipe stacked on an offshore platform ready for drilling (photo by Erin Campbell- Stone). This lab will investigate the: • different types of petroleum wells • difference between exploration and production • the costs of exploring and producing oil • the importance of understanding geology 1 of 121 The Fossil Fuels: Petroleum I - Finding Oil 5.2 Petroleum Geology 5.2.1 Petroleum Traps 5.2.1.1 Intro Petroleum is less dense than the other fluids, mainly water, that it occurs with in the subsurface. Consequently, with time, it rises upward. If it does not encounter an impermeable layer, it will rise all the way to the Earth's surface where the lighter fractions will evaporate. This produces the oil seeps and tar pits that were important sources of early petroleum products. To prevent its loss and to form an oil field, petroleum must be trapped before it reaches the surface and allowed to accumulate. This combination of natural geologic conditions is a hydrocarbon trap. Thus, oil and natural gas companies spend considerable time, effort and money looking for the right combination of geologic conditions that in the past may have produced a hydrocarbon trap. -

Zinnwald Lithium Project
Zinnwald Lithium Project Report on the Mineral Resource Prepared for Deutsche Lithium GmbH Am St. Niclas Schacht 13 09599 Freiberg Germany Effective date: 2018-09-30 Issue date: 2018-09-30 Zinnwald Lithium Project Report on the Mineral Resource Date and signature page According to NI 43-101 requirements the „Qualified Persons“ for this report are EurGeol. Dr. Wolf-Dietrich Bock and EurGeol. Kersten Kühn. The effective date of this report is 30 September 2018. ……………………………….. Signed on 30 September 2018 EurGeol. Dr. Wolf-Dietrich Bock Consulting Geologist ……………………………….. Signed on 30 September 2018 EurGeol. Kersten Kühn Mining Geologist Date: Page: 2018-09-30 2/219 Zinnwald Lithium Project Report on the Mineral Resource TABLE OF CONTENTS Page Date and signature page .............................................................................................................. 2 1 Summary .......................................................................................................................... 14 1.1 Property Description and Ownership ........................................................................ 14 1.2 Geology and mineralization ...................................................................................... 14 1.3 Exploration status .................................................................................................... 15 1.4 Resource estimates ................................................................................................. 16 1.5 Conclusions and Recommendations ....................................................................... -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Used at Rocky Flats
. TASK 1 REPORT (Rl) IDENTIFICATION OF CHEMICALS AND RADIONUCLIDES USED AT ROCKY FLATS I PROJECT BACKGROUND ChemRisk is conducting a Rocky Flats Toxicologic Review and Dose Reconstruction study for The Colorado Department of Health. The two year study will be completed by the fall of 1992. The ChemRisk study is composed of twelve tasks that represent the first phase of an independent investigation of off-site health risks associated with the operation of the Rocky Flats nuclear weapons plant northwest of Denver. The first eight tasks address the collection of historic information on operations and releases and a detailed dose reconstruction analysis. Tasks 9 through 12 address the compilation of information and communication of the results of the study. Task 1 will involve the creation of an inventory of chemicals and radionuclides that have been present at Rocky Flats. Using this inventory, chemicals and radionuclides of concern will be selected under Task 2, based on such factors as the relative toxicity of the materials, quantities used, how the materials might have been released into the environment, and the likelihood for transport of the materials off-site. An historical activities profile of the plant will be constructed under Task 3. Tasks 4, 5, and 6 will address the identification of where in the facility activities took place, how much of the materials of concern were released to the environment, and where these materials went after the releases. Task 7 addresses historic land-use in the vicinity of the plant and the location of off-site populations potentially affected by releases from Rocky Flats. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -

Flynn Creek Crater, Tennessee: Final Report, by David J
1967010060 ASTROGEOLOGIC STUDIES / ANNUAL PROGRESS REPORT " July 1, 1965 to July 1, 1966 ° 'i t PART B - h . CRATERINVESTIGATIONS N 67_1_389 N 57-" .]9400 (ACCEC_ION [4U _" EiER! (THRU} .2_ / PP (PAGLS) (CO_ w ) _5 (NASA GR OR I"MX OR AD NUMBER) (_ATEGORY) DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOQICAL SURVEY • iri i i i i iiii i i 1967010060-002 ASTROGEOLOGIC STUDIES ANNUAL PROGRESS REPORT July i, 1965 to July I, 1966 PART B: CRATER INVESTIGATIONS November 1966 This preliminary report is distributed without editorial and technical review for conformity with official standards and nomenclature. It should not be quoted without permission. This report concerns work done on behalf of the National Aeronautics and Space Administration. DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY 1967010060-003 • #' C OING PAGE ,BLANK NO/" FILMED. CONTENTS PART B--CRATER INVESTIGATIONS Page Introduction ........................ vii History and origin of the Flynn Creek crater, Tennessee: final report, by David J. Roddy .............. 1 Introductien ..................... 1 Geologic history of the Flynn Creek crater ....... 5 Origin of the Flynn Creek crater ............ ii Conc lusions ...................... 32 References cited .................... 35 Geology of the Sierra Madera structure, Texas: progress report, by H. G. Wilshire ............ 41_ Introduction ...................... 41 Stratigraphy ...................... 41 Petrography and chemical composition .......... 49 S truc ture ....................... 62 References cited ............. ...... 69 Some aspects of the Manicouagan Lake structure in Quebec, Canada, by Stephen H. Wolfe ................ 71 f Craters produced by missile impacts, by H. J. Moore ..... 79 Introduction ...................... 79 Experimental procedure ................. 80 Experimental results .................. 81 Summary ........................ 103 References cited .................... 103 Hypervelocity impact craters in pumice, by H. J. Moore and / F. -

Lithium Exploration Commences at Broken Hill
ASX ANNOUNCEMENT 3 June 2016 Lithium Exploration Commences at Broken Hill Exploration for lithium in pegmatites at Broken Hill has commenced Extensive zones of outcropping pegmatite in Silver City tenements covering some 100 square kilometres No systematic exploration for lithium ever undertaken at Broken Hill Important anomalous lithium indicator elements already identified in Waukeroo tin field Broken Hill mining centre provides logistical advantage to new discoveries Silver City Minerals Limited (ASX: SCI) (“Silver City” or “the Company”) has commenced a program of mineral exploration specifically targeting lithium hosted in pegmatites at Broken Hill (ASX Release 11 May 2016). Initial field programs have begun with systematic rock chip sampling and geological mapping of pegmatites associated with areas of tin mineralisation and evaluation of existing airborne hyperspectral data. The purpose of the program is to locate pegmatites which contain lithium minerals with a particular emphasis on spodumene (LiAlSi2O6), the dominant commercial lithium mineral. Current exploration is focussed on pegmatites located within granted exploration tenure on the northern and southern extensions of the Waukeroo tin field. SCI holds title to ten granted exploration licences and three licence applications. Next Steps SCI considers that, through systematic sampling and mineralogical studies, potential for discovery of lithium minerals at Waukeroo and elsewhere in the district is high. Work has commenced with field teams sampling and mapping, including re-sampling of drill chips from the previous tungsten resource drilling. Sampling has also been initiated in areas of the Western zone where extensive pegmatite outcrops have similarly never been assessed for lithium. SILVER CITY MINERALS LIMITED A study using an existing airborne hyperspectral (HyMap) survey is underway to assess the potential for remotely differentiating spodumene in the spectral data. -
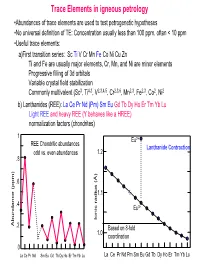
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

Celebrating 125 Years of the U.S. Geological Survey
Celebrating 125 Years of the U.S. Geological Survey Circular 1274 U.S. Department of the Interior U.S. Geological Survey Celebrating 125 Years of the U.S. Geological Survey Compiled by Kathleen K. Gohn Circular 1274 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Gale A. Norton, Secretary U.S. Geological Survey Charles G. Groat, Director U.S. Geological Survey, Reston, Virginia: 2004 Free on application to U.S. Geological Survey, Information Services Box 25286, Denver Federal Center Denver, CO 80225 For more information about the USGS and its products: Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov/ Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Gohn, Kathleen K., comp., 2004, Celebrating 125 years of the U.S. Geological Survey : U.S. Geological Survey Circular 1274, 56 p. Library of Congress Cataloging-in-Publication Data 2001051109 ISBN 0-607-86197-5 iii Message from the Today, the USGS continues respond as new environmental to map, measure, and monitor challenges and concerns emerge Director our land and its resources and and to seize new enhancements to conduct research that builds to information technology that In the 125 years since its fundamental knowledge about make producing and present- creation, the U.S. Geological the Earth, its resources, and its ing our science both easier and Survey (USGS) has provided processes, contributing relevant faster. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components.