The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primordial Hydrogen-Helium Degassing, an Overlooked Major Energy Source for Internal Terrestrial Processes
HAIT Journal of Science and Engineering B, Volume 2, Issues 1-2, pp. 125-167 Copyright C 2005 Holon Academic Institute of Technology ° Primordial hydrogen-helium degassing, an overlooked major energy source for internal terrestrial processes 1, 2 Arie Gilat ∗ and Alexander Vol 1Geological Survey of Israel (ret.), 8 Yehoash St., Jerusalem 93152, Israel 2Enlightment Ltd. 33 David Pinsky St., Haifa 34454, Israel ∗Corresponding author: [email protected] Received 20 July 2004, revised 3 February 2005, accepted 16 February 2005 Abstract The currently accepted theories concerning terrestrial processes are lacking in accounting for a source of internal energy which: (a) are quickly focused, e.g. earthquakes and volcanic eruptions; (b) are of very high density; (c) provide very high velocities of energy re- lease; (d) have very high density of the energy transport and relatively small losses during transportation over long distances; (e) are quasi- constantly released and practically limitless. This energy release is always accompanied by H- and He-degassing. Solid solutions of H and He, and compounds of He with H, O, Si and metals were discovered in laboratory experiments of ultra-high PT-conditions; He-S, He-Cl, He- C, He-N structures can be deduced from their atomic structure and compositions of natural He-reach gases. Ultra-high PT-conditions ex- ist in the Earth’s interior; hence it seems most likely that some “exotic” compounds are present in the Earth’s core and mantle. During Earth’s accretion, primordial hydrogen and helium were trapped and stored in the planet interior as H- and He- solutions and compounds, stable only under ultrahigh PT-conditions that were dis- covered in recent experiments. -

Density Difference Between Subducted Oceanic Crust and Ambient Mantle in the Mantle Transition Zone Density Difference Between S
Density Difference between Subducted Oceanic Crust and Ambient Mantle in the Mantle Transition Zone Since the beginning of plate tectonics, the were packed separately into NaCl or MgO sample oceanic lithosphere has been continually subducted chamber with a mixture of gold and MgO, and into the Earth’s deep mantle for 4.5 Gy. The compressed in the same high-pressure cell. The oceanic lithosphere consists of an upper basaltic pressure was determined by the cell volume for layer (oceanic crust) and a lower olivine-rich gold using an equation of state of gold [2], and the peridotitic layer. The total amount of subducted temperature was measured by a thermocouple. oceanic crust in this 4.5 Gy period is estimated to The cell volumes of garnet and ringwoodite were be at least ~3 × 1 0 23 kg, which is about 8% of the determined by least squares calculations using the weight of the present Earth’s mantle. Thus, the positions of X-ray diffraction peaks. The samples oceanic crust, which is rich in pyroxene and garnet, were compressed to the desired pressure at room may be the source of very important chemical temperature and heated to the maximum temperature heterogeneity in the olivine-rich Earth’s mantle. To to release non-hydrostatic stress. clarify behavior of the oceanic crust in the deep Pressure-volume-temperature data were collected mantle, accurate information about density under 47 different conditions. Figure 1 shows P -V - differences between the oceanic crust and the T data for garnet. The data collected by using an ambient mantle is very important. -

Mantle Transition Zone Structure Beneath Northeast Asia from 2-D
RESEARCH ARTICLE Mantle Transition Zone Structure Beneath Northeast 10.1029/2018JB016642 Asia From 2‐D Triplicated Waveform Modeling: Key Points: • The 2‐D triplicated waveform Implication for a Segmented Stagnant Slab fi ‐ modeling reveals ne scale velocity Yujing Lai1,2 , Ling Chen1,2,3 , Tao Wang4 , and Zhongwen Zhan5 structure of the Pacific stagnant slab • High V /V ratios imply a hydrous p s 1State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, and/or carbonated MTZ beneath 2 3 Northeast Asia Beijing, China, College of Earth Sciences, University of Chinese Academy of Sciences, Beijing, China, CAS Center for • A low‐velocity gap is detected within Excellence in Tibetan Plateau Earth Sciences, Beijing, China, 4Institute of Geophysics and Geodynamics, School of Earth the stagnant slab, probably Sciences and Engineering, Nanjing University, Nanjing, China, 5Seismological Laboratory, California Institute of suggesting a deep origin of the Technology, Pasadena, California, USA Changbaishan intraplate volcanism Supporting Information: Abstract The structure of the mantle transition zone (MTZ) in subduction zones is essential for • Supporting Information S1 understanding subduction dynamics in the deep mantle and its surface responses. We constructed the P (Vp) and SH velocity (Vs) structure images of the MTZ beneath Northeast Asia based on two‐dimensional ‐ Correspondence to: (2 D) triplicated waveform modeling. In the upper MTZ, a normal Vp but 2.5% low Vs layer compared with L. Chen and T. Wang, IASP91 are required by the triplication data. In the lower MTZ, our results show a relatively higher‐velocity [email protected]; layer (+2% V and −0.5% V compared to IASP91) with a thickness of ~140 km and length of ~1,200 km [email protected] p s atop the 660‐km discontinuity. -
![Arxiv:1906.03473V2 [Physics.Geo-Ph] 30 Dec 2019 Double the Carbon Solubility in fluids Co-Existing with Sediments Subducted Along Cool Geotherms](https://docslib.b-cdn.net/cover/0514/arxiv-1906-03473v2-physics-geo-ph-30-dec-2019-double-the-carbon-solubility-in-uids-co-existing-with-sediments-subducted-along-cool-geotherms-390514.webp)
Arxiv:1906.03473V2 [Physics.Geo-Ph] 30 Dec 2019 Double the Carbon Solubility in fluids Co-Existing with Sediments Subducted Along Cool Geotherms
Devolatilization of Subducting Slabs, Part II: Volatile Fluxes and Storage Meng Tian,1, ∗ Richard F. Katz,1 David W. Rees Jones,1, 2, 3 and Dave A. May1 1Department of Earth Sciences, University of Oxford, South Parks Road, Oxford, OX1 3AN, UK. 2Department of Earth Sciences, Bullard Laboratories, University of Cambridge, Madingley Road, Cambridge, CB3 0EZ, UK. 3School of Mathematics and Statistics, University of St Andrews, North Haugh, St Andrews, KY16 9SS, UK Subduction is a crucial part of the long-term water and carbon cycling between Earth's exo- sphere and interior. However, there is broad disagreement over how much water and carbon is liberated from subducting slabs to the mantle wedge and transported to island-arc volcanoes. In the companion paper Part I, we parameterize the metamorphic reactions involving H2O and CO2 for representative subducting lithologies. On this basis, a two-dimensional reactive transport model is constructed in this Part II. We assess the various controlling factors of CO2 and H2O release from subducting slabs. Model results show that up-slab fluid flow directions produce a flux peak of CO2 and H2O at subarc depths. Moreover, infiltration of H2O-rich fluids sourced from hydrated slab mantle enhances decarbonation or carbonation at lithological interfaces, increases slab surface fluxes, and redistributes CO2 from basalt and gabbro layers to the overlying sedimentary layer. As a result, removal of the cap sediments (by diapirism or off-scraping) leads to elevated slab surface CO2 and H2O fluxes. The modelled subduction efficiency (the percentage of initially subducted volatiles retained until ∼200 km deep) of H2O and CO2 is increased by open-system effects due to fractionation within the interior of lithological layers. -

Continental Flood Basalts Derived from the Hydrous Mantle Transition Zone
ARTICLE Received 4 Sep 2014 | Accepted 1 Jun 2015 | Published 14 Jul 2015 DOI: 10.1038/ncomms8700 Continental flood basalts derived from the hydrous mantle transition zone Xuan-Ce Wang1, Simon A. Wilde1, Qiu-Li Li2 & Ya-Nan Yang2 It has previously been postulated that the Earth’s hydrous mantle transition zone may play a key role in intraplate magmatism, but no confirmatory evidence has been reported. Here we demonstrate that hydrothermally altered subducted oceanic crust was involved in generating the late Cenozoic Chifeng continental flood basalts of East Asia. This study combines oxygen isotopes with conventional geochemistry to provide evidence for an origin in the hydrous mantle transition zone. These observations lead us to propose an alternative thermochemical model, whereby slab-triggered wet upwelling produces large volumes of melt that may rise from the hydrous mantle transition zone. This model explains the lack of pre-magmatic lithospheric extension or a hotspot track and also the arc-like signatures observed in some large-scale intracontinental magmas. Deep-Earth water cycling, linked to cold subduction, slab stagnation, wet mantle upwelling and assembly/breakup of supercontinents, can potentially account for the chemical diversity of many continental flood basalts. 1 ARC Centre of Excellence for Core to Crust Fluid Systems (CCFS), The Institute for Geoscience Research (TIGeR), Department of Applied Geology, Curtin University, GPO Box U1987, Perth, Western Australia 6845, Australia. 2 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, P.O.Box9825, Beijing 100029, China. Correspondence and requests for materials should be addressed to X.-C.W. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
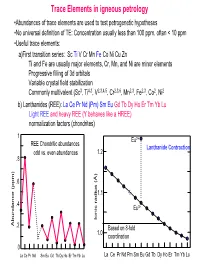
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

The Upper Mantle and Transition Zone
The Upper Mantle and Transition Zone Daniel J. Frost* DOI: 10.2113/GSELEMENTS.4.3.171 he upper mantle is the source of almost all magmas. It contains major body wave velocity structure, such as PREM (preliminary reference transitions in rheological and thermal behaviour that control the character Earth model) (e.g. Dziewonski and Tof plate tectonics and the style of mantle dynamics. Essential parameters Anderson 1981). in any model to describe these phenomena are the mantle’s compositional The transition zone, between 410 and thermal structure. Most samples of the mantle come from the lithosphere. and 660 km, is an excellent region Although the composition of the underlying asthenospheric mantle can be to perform such a comparison estimated, this is made difficult by the fact that this part of the mantle partially because it is free of the complex thermal and chemical structure melts and differentiates before samples ever reach the surface. The composition imparted on the shallow mantle by and conditions in the mantle at depths significantly below the lithosphere must the lithosphere and melting be interpreted from geophysical observations combined with experimental processes. It contains a number of seismic discontinuities—sharp jumps data on mineral and rock properties. Fortunately, the transition zone, which in seismic velocity, that are gener- extends from approximately 410 to 660 km, has a number of characteristic ally accepted to arise from mineral globally observed seismic properties that should ultimately place essential phase transformations (Agee 1998). These discontinuities have certain constraints on the compositional and thermal state of the mantle. features that correlate directly with characteristics of the mineral trans- KEYWORDS: seismic discontinuity, phase transformation, pyrolite, wadsleyite, ringwoodite formations, such as the proportions of the transforming minerals and the temperature at the discontinu- INTRODUCTION ity. -

Subduction-Transition Zone Interaction: a Review
Research Paper THEMED ISSUE: Subduction Top to Bottom 2 GEOSPHERE Subduction-transition zone interaction: A review Saskia Goes1, Roberto Agrusta1,2, Jeroen van Hunen2, and Fanny Garel3 1Department of Earth Science & Engineering, Royal School of Mines, Imperial College, London SW7 2AZ, UK GEOSPHERE; v. 13, no. 3 2Department of Earth Sciences, Durham University, Science Labs, Durham DH1 3LE, UK 3Géosciences Montpellier, Université de Montpellier, Centre national de la recherche scientifique (CNRS), 34095 Montpellier cedex 05, France doi:10.1130/GES01476.1 9 figures ABSTRACT do not; (2) on what time scales slabs are stagnant in the transition zone if they have flattened; (3) how slab-transition zone interaction is reflected in plate mo- CORRESPONDENCE: s .goes@ imperial .ac .uk As subducting plates reach the base of the upper mantle, some appear tions; and (4) how lower-mantle fast seismic anomalies can be correlated with to flatten and stagnate, while others seemingly go through unimpeded. This past subduction. CITATION: Goes, S., Agrusta, R., van Hunen, J., variable resistance to slab sinking has been proposed to affect long-term ther- Over the past 20 years since the last Subduction Top to Bottom volume and and Garel, F., 2017, Subduction-transition zone inter- action: A review: Geosphere, v. 13, no. 3, p. 1– mal and chemical mantle circulation. A review of observational constraints several reviews of subduction through the transition zone (Lay, 1994; Chris- 21, doi:10.1130/GES01476.1. and dynamic models highlights that neither the increase in viscosity between tensen, 2001; King, 2001; Billen, 2008; Fukao et al., 2009), information about upper and lower mantle (likely by a factor 20–50) nor the coincident endo- the nature of the transition zone and our understanding of how slabs dynami- Received 6 December 2016 thermic phase transition in the main mantle silicates (with a likely Clapeyron cally interact with it has increased substantially. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

Phase Transitions in Earth's Mantle and Mantle Mineralogy
Phase transitions in Earth’s Mantle and Mantle Mineralogy Upper Mantle Minerals: Olivine, Orthopyroxene, Clinopyroxene and Garnet ~13.5 GPa: Olivine Æ Wadsrlyite (DE) transition (ONSET TRANSITION ZONE) ~15.5 GPa: Pyroxene component gradually dissolve into garnet structure, resulting in the completion of pyroxene-majorite transformation >20 GPa: High CaO content in majorite is unfavorable at high pressure, leading to the formation of CaSiO3 perovskite ~24 GPa: Division of transition zone and lower mantle. Sharp transition silicate spinel to ferromagnesium silicate perovskite and magnesiowustite >24 GPa: Most of Al2O3 resides in majorite at transition zone pressures, a transformation from Majorite to Al-bearing orthorhombic perovskite completes at pressure higher than that of post-spinel transformation Lower Mantle Minerals: Orthorhobic perovskite, Magnesiowustite, CaSiO3 perovskite ~27 GPa: Transformation of Al and Si rich basalt to perovskite lithology with assemblage of Al-bearing perovskite, CaSiO3, stishovite and Al-phases Upper Mantle: olivine, garnet and pyroxene Transition zone: olivine (a-phase) transforms to wadsleyite (b-phase) then to spinel structure (g-phase) and finally to perovskite + magnesio-wüstite. Transformations occur at P and T conditions similar to 410, 520 and 660 km seismic discontinuities Xenoliths: (mantle fragments brought to surface in lavas) 60% Olivine + 40 % Pyroxene + some garnet Images removed due to copyright considerations. Garnet: A3B2(SiO4)3 Majorite FeSiO4, (Mg,Fe)2 SiO4 Germanates (Co-, Ni- and Fe-