Durham Research Online
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formation of the Lower Ocean Crust and the Crystallization of Gabbroic Cumulates at a Very Slowly Spreading Ridge
Journal of Volcanology and Geothermal Research 110 ?2001) 191±233 www.elsevier.com/locate/jvolgeores Formation of the lower ocean crust and the crystallization of gabbroic cumulates at a very slowly spreading ridge James H. Natlanda,*, Henry J.B. Dickb aRosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, FL 33149, USA bWoods hole Oceanographic Institution, Woods hole, MA 02543, USA Revised 25 June 2000 Abstract Ocean Drilling Program Hole 735B was extended to 1508 m below the sea ¯oor during Leg 176, atop a shallow banknear Atlantis II Fracture Zone on the very slowly spreading Southwest Indian Ridge. All the drilling was in gabbro, and recovery averaged nearly 87%. The drill penetrated a series of stacked plutons consisting mostly of olivine gabbro, but some with troctolite. Each pluton is some 200±500 m thick, each has its own internally coherent stratigraphy, and each apparently represents an individual event of signi®cant magma in¯ation and addition to the crust. The entire column was extensively deformed along inclined zones of distributed shear before it was completely frozen, this marking the onset of unroo®ng of the rocks and their ascent to high rift mountains. The deformation mobilized late-stage melts into ¯ow patterns which led to concentration of ilmenite and magnetite in hundreds of seams of oxide gabbro along or near zones of strong crystal±plastic deformation, the highly differentiated melts overall being concentrated by buoyancy forces toward the top of the section, especially in one zone nearly 70 m thick. However, upward ¯ow was ultimately blocked or de¯ected by zones of impermeable rockresulting either from downward freezing or grain-size reduction during shear. -

Small Volume Andesite Magmas and Melt–Mush Interactions at Ruapehu, New Zealand: Evidence from Melt Inclusions
Contrib Mineral Petrol DOI 10.1007/s00410-013-0880-7 ORIGINAL PAPER Small volume andesite magmas and melt–mush interactions at Ruapehu, New Zealand: evidence from melt inclusions Geoff Kilgour • Jon Blundy • Kathy Cashman • Heidy M. Mader Received: 1 November 2012 / Accepted: 12 April 2013 Ó Springer-Verlag Berlin Heidelberg 2013 Abstract Historical eruptions from Mt. Ruapehu (New From these data, we infer that individual melt batches rise Zealand) have been small (\0.001 km3 of juvenile magma) through, and interact with, crystal mush zones formed by and have often occurred without significant warning. antecedent magmas. From this perspective, we envision the Developing better modelling tools requires an improved magmatic system at Ruapehu as frequently recharged by understanding of the magma storage and transport system small magma inputs that, in turn, cool and crystallise to beneath the volcano. Towards that end, we have analysed varying degrees. Melts that are able to erupt through this the volatile content and major element chemistry of network of crystal mush entrain (to a greater or lesser groundmass glass and phenocryst-hosted melt inclusions in extent) exotic crystals. In the extreme case (such as the 1996 erupted samples from 1945 to 1996. We find that during this eruption), the resulting scoria contain melt inclusion-bear- time period, magma has been stored at depths of *2–9 km, ing crystals that are exotic to the transporting magma. consistent with inferences from geophysical data. Our data Finally, we suggest that complex interactions between also show that Ruapehu magmas are relatively H2O-poor recharge and antecedent magmas are probably common, but (\2 wt%) and CO2-rich (B1,000 ppm) compared to typical that the small volumes and short time scales of recharge at arc andesites. -

Large Igneous Provinces: a Driver of Global Environmental and Biotic Changes, Geophysical Monograph 255, First Edition
2 Radiometric Constraints on the Timing, Tempo, and Effects of Large Igneous Province Emplacement Jennifer Kasbohm1, Blair Schoene1, and Seth Burgess2 ABSTRACT There is an apparent temporal correlation between large igneous province (LIP) emplacement and global envi- ronmental crises, including mass extinctions. Advances in the precision and accuracy of geochronology in the past decade have significantly improved estimates of the timing and duration of LIP emplacement, mass extinc- tion events, and global climate perturbations, and in general have supported a temporal link between them. In this chapter, we review available geochronology of LIPs and of global extinction or climate events. We begin with an overview of the methodological advances permitting improved precision and accuracy in LIP geochro- nology. We then review the characteristics and geochronology of 12 LIP/event couplets from the past 700 Ma of Earth history, comparing the relative timing of magmatism and global change, and assessing the chronologic support for LIPs playing a causal role in Earth’s climatic and biotic crises. We find that (1) improved geochronol- ogy in the last decade has shown that nearly all well-dated LIPs erupted in < 1 Ma, irrespective of tectonic set- ting; (2) for well-dated LIPs with correspondingly well-dated mass extinctions, the LIPs began several hundred ka prior to a relatively short duration extinction event; and (3) for LIPs with a convincing temporal connection to mass extinctions, there seems to be no single characteristic that makes a LIP deadly. Despite much progress, higher precision geochronology of both eruptive and intrusive LIP events and better chronologies from extinc- tion and climate proxy records will be required to further understand how these catastrophic volcanic events have changed the course of our planet’s surface evolution. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Petrology of the Noritic and Gabbronoritic Rocks Below the J-M Reef in the Mountain View Area, Stillwater Complex, Montana
Petrology of the Noritic and Gabbronoritic Rocks below the J-M Reef in the Mountain View Area, Stillwater Complex, Montana U.S. GEOLOGICAL SURVEY BULLETIN 1674-C Chapter C Petrology of the Noritic and Gabbronoritic Rocks below the J-M Reef in the Mountain View Area, Stillwater Complex, Montana By NORMAN J PAGE and BARRY C. MORING U.S. GEOLOGICAL SURVEY BULLETIN 1674 CONTRIBUTIONS ON ORE DEPOSITS IN THE EARLY MAGMATIC ENVIRONMENT DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1990 For sale by the Books and Open-File Reports Section, U.S. Geological Survey Federal Center, Box 25425 Denver, CO 80225 Library of Congress Cataloging-in-Publication Data Page, Norman, J Petrology of the noritic and gabbronoritic rocks below the J-M Reef in the Mountain View area, Stillwater Complex, Montana / by Norman J Page and Barry C. Moring. p. cm. (U.S. Geological Survey bulletin ; 1674-C) (Contributions on ore deposits in the early magmatic environment Includes bibliographical references. Supt. of Docs, no.: I 19.3: 1674-C 1. Petrology Beartooth Mountains Region (Mont, and Wyo.) 2. Geolo gy Beartooth Mountains Region (Mont, and Wyo.) I. Moring, Barry C. II. Title. III. Title: Stillwater Complex, Montana. IV. Series. V. Series: Contri butions on ore deposits in the early magmatic environment ; ch. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
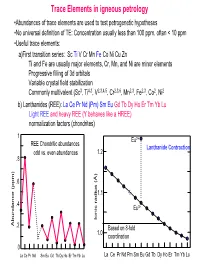
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Connecting the Deep Earth and the Atmosphere
In Mantle Convection and Surface Expression (Cottaar, S. et al., eds.) AGU Monograph 2020 (in press) Connecting the Deep Earth and the Atmosphere Trond H. Torsvik1,2, Henrik H. Svensen1, Bernhard Steinberger3,1, Dana L. Royer4, Dougal A. Jerram1,5,6, Morgan T. Jones1 & Mathew Domeier1 1Centre for Earth Evolution and Dynamics (CEED), University of Oslo, 0315 Oslo, Norway; 2School of Geosciences, University of Witwatersrand, Johannesburg 2050, South Africa; 3Helmholtz Centre Potsdam, GFZ, Telegrafenberg, 14473 Potsdam, Germany; 4Department of Earth and Environmental Sciences, Wesleyan University, Middletown, Connecticut 06459, USA; 5DougalEARTH Ltd.1, Solihull, UK; 6Visiting Fellow, Earth, Environmental and Biological Sciences, Queensland University of Technology, Brisbane, Queensland, Australia. Abstract Most hotspots, kimberlites, and large igneous provinces (LIPs) are sourced by plumes that rise from the margins of two large low shear-wave velocity provinces in the lowermost mantle. These thermochemical provinces have likely been quasi-stable for hundreds of millions, perhaps billions of years, and plume heads rise through the mantle in about 30 Myr or less. LIPs provide a direct link between the deep Earth and the atmosphere but environmental consequences depend on both their volumes and the composition of the crustal rocks they are emplaced through. LIP activity can alter the plate tectonic setting by creating and modifying plate boundaries and hence changing the paleogeography and its long-term forcing on climate. Extensive blankets of LIP-lava on the Earth’s surface can also enhance silicate weathering and potentially lead to CO2 drawdown (cooling), but we find no clear relationship between LIPs and post-emplacement variation in atmospheric CO2 proxies on very long (>10 Myrs) time- scales. -

Attributes of Skaergaard-Type PGE Reefs
Attributes of Skaergaard-Type PGE Reefs James D. Miller, Jr.1 and Jens C. Ø. Andersen2 1Minnesota Geological Survey, c/o NRRI, University of Minnesota-Duluth, 5013 Miller Trunk Hwy., Duluth, MN, 55811 , USA 2Camborne School of Mines, University of Exeter, Redruth, Cornwall, TR15 3SE UK e-mail: [email protected], [email protected] Stratiform, platinum group element (PGE) peridotite, pyroxenite, and dunite are usually deposits have long been known to occur in quantitatively minor. When well-differentiated, ultramafic-mafic intrusive complexes such as such intrusions display a simplified cumulus Bushveld and Stillwater (Naldrett, 1989b). stratigraphy following the scheme: Ol or Pl only-> Commonly known as PGE reefs, such deposits are Ol + Pl -> Pl + Cpx + FeOx ± Ol -> Pl + Cpx + typically found near the transition from ultramafic FeOx + Ol + Ap. They have a strong cryptic to mafic cumulates where they occur as 1-3 meter layering towards iron-enrichment indicative of thick intervals enriched in PGEs (1-20 ppm) and Fenner-type differentiation. They differ from trace to moderate amounts of sulfide (0.5-5 wt %). classic PGE reef-bearing intrusions by lacking a However, relatively recent discoveries have significant ultramafic component (early olivine- demonstrated that potentially economic stratiform only crystallization is minor to absent in most PGE mineralization may also occur in tholeiitic tholeiitic intrusions) and by being poor in mafic layered intrusions. Au- and PGE-bearing orthopyroxene (inverted pigeonite is rarely a layers -

The East Greenland Rifted Volcanic Margin
GEOLOGICAL SURVEY OF DENMARK AND GREENLAND BULLETIN 24 • 2011 The East Greenland rifted volcanic margin C. Kent Brooks GEOLOGICAL SURVEY OF DENMARK AND GREENLAND DANISH MINISTRY OF CLIMATE, ENERGY AND BUILDING 1 Geological Survey of Denmark and Greenland Bulletin 24 Keywords East Greenland, North Atlantic, rifted volcanic margin, large igneous province, LIP, Palaeogene, basalt, syenite, nephelinite, carbona- tite, uplift. Cover Sundown over the nunataks in the Main Basalts (Skrænterne Fm) to the south of Scoresby Sund. Camped on the glacier, the 1965 Ox- ford University East Greenland Expedition travelled and collected from this area on foot, manhauling equipment on the sledge to the left. The expedition results were published in Fawcett et al. (1973). Frontispiece: facing page Mountains of horizontally layered basalt flows rising to about 2000 m on the south side of Scoresby Sund. Typical trap topography as found throughout most of the Kangerlussuaq–Scoresby Sund inland area. Chief editor of this series: Adam A. Garde Editorial board of this series: John A. Korstgård, Department of Geoscience, Aarhus University; Minik Rosing, Geological Museum, University of Copenhagen; Finn Surlyk, Department of Geography and Geology, University of Copenhagen Scientific editor of this volume: Adam A. Garde Editorial secretaries: Jane Holst and Esben W. Glendal Referees: Dennis K. Bird (USA) and Christian Tegner (DK) Illustrations: Eva Melskens with contributions from Adam A. Garde Digital photographic work: Benny Schark Graphic production: Kristian A. Rasmussen Printers: Rosendahls · Schultz Grafisk A/S, Albertslund, Denmark Manuscript received: 1 March 2011 Final version approved: 20 September 2011 Printed: 22 December 2011 ISSN 1604-8156 ISBN 978-87-7871-322-3 Citation of the name of this series It is recommended that the name of this series is cited in full, viz. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

Exsolved Volatiles in Magma Reservoirs 4 5 Marie Edmonds1* and Andrew W
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Apollo 1 JVGR review paper 2 3 Exsolved Volatiles in Magma Reservoirs 4 5 Marie Edmonds1* and Andrew W. Woods1,2 6 7 1 Earth Sciences Department, University of Cambridge, Downing Street, Cambridge CB2 3EQ, UK 8 2 BP Institute, Bullard Laboratories, Madingley Rd, Cambridge CB3 0EZ, UK 9 10 *Corresponding author, [email protected] 11 12 1. Introduction 13 2. Achieving volatile saturation in magmas 14 2.1. Volatiles in natural magmatic systems 15 2.2. Sulfur partitioning into the exsolved volatile phase 16 3. Consequences of exsolved volatiles in magma reservoirs for rheology and dynamics 17 3.1. Magma compressibility 18 3.2. Pressure increase during second boiling 19 3.3. Effect of compressibility on the mass of magma erupted 20 4. The role of the exsolved volatile phase in magma mixing and mingling 21 4.1. Magma mixing and overturn, driven by vesiculation of the lower magma layer 22 4.2. Vesiculation of underplating magmas and formation of mafic enclaves 23 5. Exsolved volatile phase generation and transport through crystal-rich magma bodies 24 5.1. Transport of an exsolved volatile phase in crystal-rich magma 25 5.2. Implications of our new understanding of the transport of the exsolved volatile phase 26 through crystalline magmas for ore deposits 27 6. Summary and problems for the future 28 29 30 31 32 33 34 1 35 Abstract 36 We review our understanding of the exsolved volatile phase co-existing with magmas during pre- 37 eruptive storage at the pressures and temperatures corresponding to crustal magma reservoirs.