Jia Guo A, Hui Li A, Di Wang A, Liugen Zhang A, Yuhua Maa, Naeem Akram A, Yi Zhang B, Jide Wang A,*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
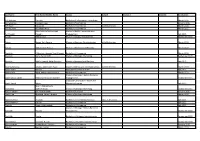
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation A/L Krishnan Thiinash Bachelor of Information Technology March 2015 A/L Selvaraju Theeban Raju Bachelor of Commerce January 2015 A/P Balan Durgarani Bachelor of Commerce with Distinction March 2015 A/P Rajaram Koushalya Priya Bachelor of Commerce March 2015 Hiba Mohsin Mohammed Master of Health Leadership and Aal-Yaseen Hussein Management July 2015 Aamer Muhammad Master of Quality Management September 2015 Abbas Hanaa Safy Seyam Master of Business Administration with Distinction March 2015 Abbasi Muhammad Hamza Master of International Business March 2015 Abdallah AlMustafa Hussein Saad Elsayed Bachelor of Commerce March 2015 Abdallah Asma Samir Lutfi Master of Strategic Marketing September 2015 Abdallah Moh'd Jawdat Abdel Rahman Master of International Business July 2015 AbdelAaty Mosa Amany Abdelkader Saad Master of Media and Communications with Distinction March 2015 Abdel-Karim Mervat Graduate Diploma in TESOL July 2015 Abdelmalik Mark Maher Abdelmesseh Bachelor of Commerce March 2015 Master of Strategic Human Resource Abdelrahman Abdo Mohammed Talat Abdelziz Management September 2015 Graduate Certificate in Health and Abdel-Sayed Mario Physical Education July 2015 Sherif Ahmed Fathy AbdRabou Abdelmohsen Master of Strategic Marketing September 2015 Abdul Hakeem Siti Fatimah Binte Bachelor of Science January 2015 Abdul Haq Shaddad Yousef Ibrahim Master of Strategic Marketing March 2015 Abdul Rahman Al Jabier Bachelor of Engineering Honours Class II, Division 1 -
![UNIT 1 味道怎么样? [Wèi-Dào Zěn-Me Yàng?] How Is the Taste?](https://docslib.b-cdn.net/cover/4270/unit-1-w%C3%A8i-d%C3%A0o-z%C4%9Bn-me-y%C3%A0ng-how-is-the-taste-554270.webp)
UNIT 1 味道怎么样? [Wèi-Dào Zěn-Me Yàng?] How Is the Taste?
1 UNIT 1 味道怎么样? [wèi-dào zěn-me yàng?] How is the taste? The following is a conversation between the waitress at 好好茶餐廳 and a customer whom just arrived at the restaurant. : 你好,请问⼏位?(nǐ*-hǎo, qǐng-wèn jǐ wèi?) 你好,请问⼏位?(nei5-hou2, cing2-man6 gei2-wai2?) Hi, How many people please? : 两位,谢谢。(liǎng-wèi, xiè-xie) 两位,唔该。 (loeng5-wai2, m4-goi1) Two people, please. 我们已经订位了。(wǒ-men yǐ-jīng dìng-wèi le) 我哋已经订咗位。(ngo5-dei6 ji5-ging1 deng6 zo2 wai2) We already made a reservation. : 好的。请问你叫什么名字?(hǎo-de. qǐng-wèn nǐ jiào shén-me míng-zì?) 好。请问你叫咩名呀?(hou2. cing2-man6 nei5 giu3 me1 meng2 aa3?) Okay. What is your name, please? : 我姓叶。(wǒ xìng yè) 我姓叶。(ngo5 sing3 jip6) My last name is Yip. : 我找到了,两位请跟我来。(wǒ* zhǎo dào le, liǎng wèi qǐng gēn wǒ lái) 我搵到喇。两位请跟我嚟。(ngo5 wan2 dou2 laa3. loeng5-wai2 cing2 gan1 ngo5 lei4) Original material under copyright, 2020 Jade Jia Ying Wu 2 I found it, please come follow me. : 请问两位想喝什么? (qǐng-wèn liǎng-wèi xiǎng hē shén-me?) 请问两位想饮咩呀?(cing2-man6 loeng5-wai2 soeng2 jam2 me1 aa3?) What would you two like to drink? : 我想要⼀杯港式奶茶。(wǒ* xiǎng yào yì-bēi gǎng shì nǎi-chá) 我想要⼀杯港式奶茶。(ngo5 soeng2 jiu3 jat1-bui1 gong2-sik1 naai5-caa4) I would like a Hong Kong-style milk tea. : 我要⽔就⾏了。(wǒ yào shuǐ jiù xíng le) 我要⽔就得喇。(ngo5 jiu3 seoi2 zau6 dak1 laa3) Water is fine for me. : 你们想吃什么?(nǐ-men xiǎng chī shén-me?) 你哋想⾷咩呀?(nei5-dei6 soeng2 sik6 me1 aa3?) What would you like to eat? : 我想要⼀个鲜虾馄饨⾯。(wǒ xiǎng* yào yí-gè xiān xiā hún-tūn miàn) 我想要⼀個鲜虾馄饨⾯。(ngo5 soeng2 jiu3 jat1-go3 sin1 haa1 wan4-tan1 min6) I would like a shrimp wonton noodle soup. -

中国人的姓名 王海敏 Wang Hai Min
中国人的姓名 王海敏 Wang Hai min last name first name Haimin Wang 王海敏 Chinese People’s Names Two parts Last name First name 姚明 Yao Ming Last First name name Jackie Chan 成龙 cheng long Last First name name Bruce Lee 李小龙 li xiao long Last First name name The surname has roughly several origins as follows: 1. the creatures worshipped in remote antiquity . 龙long, 马ma, 牛niu, 羊yang, 2. ancient states’ names 赵zhao, 宋song, 秦qin, 吴wu, 周zhou 韩han,郑zheng, 陈chen 3. an ancient official titles 司马sima, 司徒situ 4. the profession. 陶tao,钱qian, 张zhang 5. the location and scene in residential places 江jiang,柳 liu 6.the rank or title of nobility 王wang,李li • Most are one-character surnames, but some are compound surname made up of two of more characters. • 3500Chinese surnames • 100 commonly used surnames • The three most common are 张zhang, 王wang and 李li What does my name mean? first name strong beautiful lively courageous pure gentle intelligent 1.A person has an infant name and an official one. 2.In the past,the given names were arranged in the order of the seniority in the family hierarchy. 3.It’s the Chinese people’s wish to give their children a name which sounds good and meaningful. Project:Search on-Line www.Mandarinintools.com/chinesename.html Find Chinese Names for yourself, your brother, sisters, mom and dad, or even your grandparents. Find meanings of these names. ----What is your name? 你叫什么名字? ni jiao shen me ming zi? ------ 我叫王海敏 wo jiao Wang Hai min ------ What is your last name? 你姓什么? ni xing shen me? (你贵姓?)ni gui xing? ------ 我姓 王,王海敏。 wo xing wang, Wang Hai min ----- What is your nationality? 你是哪国人? ni shi na guo ren? ----- I am chinese/American 我是中国人/美国人 Wo shi zhong guo ren/mei guo ren 百家 姓 bai jia xing 赵(zhào) 钱(qián) 孙(sūn) 李(lǐ) 周(zhōu) 吴(wú) 郑(zhèng) 王(wán 冯(féng) 陈(chén) 褚(chǔ) 卫(wèi) 蒋(jiǎng) 沈(shěn) 韩(hán) 杨(yáng) 朱(zhū) 秦(qín) 尤(yóu) 许(xǔ) 何(hé) 吕(lǚ) 施(shī) 张(zhāng). -

Ideophones in Middle Chinese
KU LEUVEN FACULTY OF ARTS BLIJDE INKOMSTSTRAAT 21 BOX 3301 3000 LEUVEN, BELGIË ! Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas'Van'Hoey' ' Presented(in(fulfilment(of(the(requirements(for(the(degree(of(( Master(of(Arts(in(Linguistics( ( Supervisor:(prof.(dr.(Jean=Christophe(Verstraete((promotor)( ( ( Academic(year(2014=2015 149(431(characters Abstract (English) Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas Van Hoey This M.A. thesis investigates ideophones in Tang dynasty (618-907 AD) Middle Chinese (Sinitic, Sino- Tibetan) from a typological perspective. Ideophones are defined as a set of words that are phonologically and morphologically marked and depict some form of sensory image (Dingemanse 2011b). Middle Chinese has a large body of ideophones, whose domains range from the depiction of sound, movement, visual and other external senses to the depiction of internal senses (cf. Dingemanse 2012a). There is some work on modern variants of Sinitic languages (cf. Mok 2001; Bodomo 2006; de Sousa 2008; de Sousa 2011; Meng 2012; Wu 2014), but so far, there is no encompassing study of ideophones of a stage in the historical development of Sinitic languages. The purpose of this study is to develop a descriptive model for ideophones in Middle Chinese, which is compatible with what we know about them cross-linguistically. The main research question of this study is “what are the phonological, morphological, semantic and syntactic features of ideophones in Middle Chinese?” This question is studied in terms of three parameters, viz. the parameters of form, of meaning and of use. -

JIA LIU LSK4051, Hong Kong University of Science & Technology Clear Water Bay, Hong Kong [email protected] +852 2358-7709 (Updated September 2020)
JIA LIU LSK4051, Hong Kong University of Science & Technology Clear Water Bay, Hong Kong [email protected] +852 2358-7709 (Updated September 2020) EDUCATION Columbia University, New York, NY Ph.D. in Marketing, 2017 M.S. in Marketing, 2011 Michigan State University, East Lansing, MI M.S. in Statistics, 2010 Tianjin University, Tianjin, China B.S. in Mathematics, 2008 ACADEMIC POSITIONS Hong Kong University of Science & Technology, HK Assistant Professor in Marketing, Aug. 2018 - present PROFESSIONAL EXPERIENCE Microsoft Research, New York, NY Postdoctoral Researcher, mentored by Duncan Watts, Aug. 2017 - June 2018 Research Intern, mentored by Shawndra Hill, May - July, 2016 Consulting Researcher, Computational Social Science Group, February - April, 2016 Advertising Research Foundation, New York, NY Research Intern, under the supervision of Dr. William Cook, May-Aug. 2011 RESEARCH INTERESTS Substantive: Consumer Online Search, Search Engine Marketing, TV Advertising, Cross- Channel Advertising, Loyalty Programs, Behavioral Economics, Recommendation Sys- tem, User Generated Content, Social Media Methodological: Topic Modeling, Natural Language Processing, Machine Learning, Deep Neural Network, Bayesian Methods, Causal Inference, Lab/Field Experiments Jia Liu j 1 PUBLICATIONS(∗INDICATES EQUAL AUTHORSHIP) Liu, Jia, Olivier Toubia, and Shawndra Hill (2020), “Content-based Model of Web Search Behavior: An Application to TV Show Search.” forthcoming at Management Science. - Best paper award at the 2018 China Marketing International Conference Liu, Jia, and Asim Ansari (2020), “Understanding Consumer Dynamic Decision Making Un- der Competing Loyalty Programs.” Journal of Marketing Research, 57(3), 422-444. [Link] Liu, Jia∗, and Olivier Toubia∗ (2020), “Search Query Formation by Strategic Consumers.” Quantitative Marketing and Economics, 18, 155-194. [Link] Liu, Jia, and Olivier Toubia (2018), “A Semantic Approach for Estimating Consumer Content Preferences from Online Search Queries.” Marketing Science, 37 (6), 930-952. -

Using Online Applications to Improve Tone Perception Among L2 Learners of Chinese (网络应用对中文二语学习者声调辨识的有效性研究)
Journal of Technology and Chinese Language Teaching Volume 10 Number 1, June 2019 http://www.tclt.us/journal/2019v10n1/xulili.pdf pp. 26-56 Using Online Applications to Improve Tone Perception among L2 Learners of Chinese (网络应用对中文二语学习者声调辨识的有效性研究) Xu, Hongying Li, Yan Li, Yingjie (徐红英) (李艳) (李颖颉) University of University of University of Colorado- Wisconsin-La Crosse Kansas Boulder (威斯康星大学拉克 (堪萨斯大学) (科罗拉多大学博尔得分 罗校区) [email protected] 校) [email protected] [email protected] Abstract: This study investigated the effectiveness of an online application in helping beginning-level Chinese learners improve their perception of the tones in Mandarin Chinese. Two groups—one experimental and one traditional—of beginning Chinese learners from two universities in the Midwest participated in this study. The experimental group (n=20) used the online application to practice tones for four 15-minute sessions in class. The traditional group (n=11) participated in traditional instructor-led practice in class in lieu of the online practice. Both groups completed a pre-test, an immediately administered post-test, and a delayed post-test designed to assess their perception of the tones of monosyllabic and disyllabic words. No statistically significant difference has been found between the two groups in their tone perception performance in the post-test and in the delayed post-test. However, the experimental group showed a positive trend in improving their perception on those tones which posed more difficulty than others. Their experience with this online application and the pronunciation learning strategies of participants in the experimental group were also examined through a survey. Based on the findings, it is proposed that the use of online tone practice is worthwhile in a Chinese language class, but might fit better into the curriculum as external assignments. -

Names of Chinese People in Singapore
101 Lodz Papers in Pragmatics 7.1 (2011): 101-133 DOI: 10.2478/v10016-011-0005-6 Lee Cher Leng Department of Chinese Studies, National University of Singapore ETHNOGRAPHY OF SINGAPORE CHINESE NAMES: RACE, RELIGION, AND REPRESENTATION Abstract Singapore Chinese is part of the Chinese Diaspora.This research shows how Singapore Chinese names reflect the Chinese naming tradition of surnames and generation names, as well as Straits Chinese influence. The names also reflect the beliefs and religion of Singapore Chinese. More significantly, a change of identity and representation is reflected in the names of earlier settlers and Singapore Chinese today. This paper aims to show the general naming traditions of Chinese in Singapore as well as a change in ideology and trends due to globalization. Keywords Singapore, Chinese, names, identity, beliefs, globalization. 1. Introduction When parents choose a name for a child, the name necessarily reflects their thoughts and aspirations with regards to the child. These thoughts and aspirations are shaped by the historical, social, cultural or spiritual setting of the time and place they are living in whether or not they are aware of them. Thus, the study of names is an important window through which one could view how these parents prefer their children to be perceived by society at large, according to the identities, roles, values, hierarchies or expectations constructed within a social space. Goodenough explains this culturally driven context of names and naming practices: Department of Chinese Studies, National University of Singapore The Shaw Foundation Building, Block AS7, Level 5 5 Arts Link, Singapore 117570 e-mail: [email protected] 102 Lee Cher Leng Ethnography of Singapore Chinese Names: Race, Religion, and Representation Different naming and address customs necessarily select different things about the self for communication and consequent emphasis. -

The Hidden Pandemic of Family Violence During COVID-19: Unsupervised Learning of Tweets
JOURNAL OF MEDICAL INTERNET RESEARCH Xue et al Original Paper The Hidden Pandemic of Family Violence During COVID-19: Unsupervised Learning of Tweets Jia Xue1,2, PhD; Junxiang Chen3, PhD; Chen Chen4, PhD; Ran Hu1, MA, MSW; Tingshao Zhu5, PhD 1Factor-Inwentash Faculty of Social Work, University of Toronto, Toronto, ON, Canada 2Faculty of Information, University of Toronto, Toronto, ON, Canada 3School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States 4Middleware System Research Group, University of Toronto, Toronto, ON, Canada 5Institute of Psychology, Chinese Academy of Sciences, Beijing, China Corresponding Author: Jia Xue, PhD Factor-Inwentash Faculty of Social Work University of Toronto 246 Bloor St W Toronto, ON, M5S 1V4 Canada Phone: 1 416 946 5429 Email: [email protected] Abstract Background: Family violence (including intimate partner violence/domestic violence, child abuse, and elder abuse) is a hidden pandemic happening alongside COVID-19. The rates of family violence are rising fast, and women and children are disproportionately affected and vulnerable during this time. Objective: This study aims to provide a large-scale analysis of public discourse on family violence and the COVID-19 pandemic on Twitter. Methods: We analyzed over 1 million tweets related to family violence and COVID-19 from April 12 to July 16, 2020. We used the machine learning approach Latent Dirichlet Allocation and identified salient themes, topics, and representative tweets. Results: We extracted 9 themes from 1,015,874 tweets on family -

The Whole Process Cost Management Based on BIM Jixue Zou, Jia Guo
Advances in Social Science, Education and Humanities Research, volume 120 World Conference on Management Science and Human Social Development (MSHSD 2017) The whole process cost management based on BIM jixue Zou, jia Guo, jing Wu No.8, dongyi road, chang 'an district, xi 'an city, shaanxi province China Building 10-13, building no.58, south second ring, xi 'an city China 277 yantaxi road, xi 'an city, shaanxi province China [email protected] Keywords:The project cost ,BIM , Management Abstract. The whole process cost management is to ensure that the investment benefit of construction projects, from feasibility study on construction project started by the preliminary design, expand the preliminary design, construction design, contracting, construction, commissioning, completion, production and final accounts, after the evaluation of the overall process, all around the engineering cost of business behavior and organizational activities.This paper introduces how the cost management of BIM technology plays a role in the project construction. 1 Introduction At present, China's construction project is in the whole process cost management mode. Engineering cost control is divided into decision-making stage, implementation stage (design, construction) and operation stage according to the stage of engineering formation. Corresponding to it is estimation, budget estimate, contract price, construction budget, payment of progress payment, completion settlement, etc. Engineering cost management every link data volume is quite large, the calculation -

A Comparison of the Korean and Japanese Approaches to Foreign Family Names
15 A Comparison of the Korean and Japanese Approaches to Foreign Family Names JIN Guanglin* Abstract There are many foreign family names in Korean and Japanese genealogies. This paper is especially focused on the fact that out of approximately 280 Korean family names, roughly half are of foreign origin, and that out of those foreign family names, the majority trace their beginnings to China. In Japan, the Newly Edited Register of Family Names (新撰姓氏錄), published in 815, records that out of 1,182 aristocratic clans in the capital and its surroundings, 326 clans—approximately one-third—originated from China and Korea. Does the prevalence of foreign family names reflect migration from China to Korea, and from China and Korea to Japan? Or is it perhaps a result of Korean Sinophilia (慕華思想) and Japanese admiration for Korean and Chinese cultures? Or could there be an entirely distinct explanation? First I discuss premodern Korean and ancient Japanese foreign family names, and then I examine the formation and characteristics of these family names. Next I analyze how migration from China to Korea, as well as from China and Korea to Japan, occurred in their historical contexts. Through these studies, I derive answers to the above-mentioned questions. Key words: family names (surnames), Chinese-style family names, cultural diffusion and adoption, migration, Sinophilia in traditional Korea and Japan 1 Foreign Family Names in Premodern Korea The precise number of Korean family names varies by record. The Geography Annals of King Sejong (世宗實錄地理志, 1454), the first systematic register of Korean family names, records 265 family names, but the Survey of the Geography of Korea (東國輿地勝覽, 1486) records 277. -

Methodology of Adaptive Prognostics and Health Management in Dynamic Work Environment
Methodology of Adaptive Prognostics and Health Management in Dynamic Work Environment A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy In the Department of Mechanical and Materials Engineering of the College of Engineering and Applied Science by Jianshe Feng June 2020 B.Sc. in Mechanical Engineering, Tongji University (2012) M.Sc. in Mechatronics Engineering, Zhejiang University (2015) Committee: Prof. Jay Lee (Chair) Prof. Jing Shi Prof. Manish Kumar Prof. Thomas Huston Dr. Hossein Davari Dr. Zongchang Liu ii Abstract Prognostics and health management (PHM) has gradually become an essential technique to improve the availability and efficiency of a complex system. With the rapid advance- ment of sensor technology and communication technology, a huge amount of real-time data are generated from various industrial applications, which brings new challenges to PHM in the context of big data streams. On one hand, high-volume stream data places a heavy demand on data storage, communication, and PHM modeling. On the other hand, continuous change and drift are essential properties of stream data in an evolving environment, which requires the PHM model to be capable to capture the new information in stream data adaptively, efficiently and continuously. This research proposes a systematic methodology to develop an effective online learning PHM with adaptive sampling techniques to fuse information from continuous stream data. An adaptive sample selection strategy is developed so that the representative samples can be effectively selected in both off-line and online environment. In addition, various data-driven models, including probabilistic models, Bayesian algorithms, incremental methods, and ensemble algorithms, are employed and integrated into the proposed methodology for model establishment and updating with important samples selected from streaming sequence. -

A Study of Xu Xu's Ghost Love and Its Three Film Adaptations THESIS
Allegories and Appropriations of the ―Ghost‖: A Study of Xu Xu‘s Ghost Love and Its Three Film Adaptations THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Arts in the Graduate School of The Ohio State University By Qin Chen Graduate Program in East Asian Languages and Literatures The Ohio State University 2010 Master's Examination Committee: Kirk Denton, Advisor Patricia Sieber Copyright by Qin Chen 2010 Abstract This thesis is a comparative study of Xu Xu‘s (1908-1980) novella Ghost Love (1937) and three film adaptations made in 1941, 1956 and 1995. As one of the most popular writers during the Republican period, Xu Xu is famous for fiction characterized by a cosmopolitan atmosphere, exoticism, and recounting fantastic encounters. Ghost Love, his first well-known work, presents the traditional narrative of ―a man encountering a female ghost,‖ but also embodies serious psychological, philosophical, and even political meanings. The approach applied to this thesis is semiotic and focuses on how each text reflects the particular reality and ethos of its time. In other words, in analyzing how Xu‘s original text and the three film adaptations present the same ―ghost story,‖ as well as different allegories hidden behind their appropriations of the image of the ―ghost,‖ the thesis seeks to broaden our understanding of the history, society, and culture of some eventful periods in twentieth-century China—prewar Shanghai (Chapter 1), wartime Shanghai (Chapter 2), post-war Hong Kong (Chapter 3) and post-Mao mainland (Chapter 4). ii Dedication To my parents and my husband, Zhang Boying iii Acknowledgments This thesis owes a good deal to the DEALL teachers and mentors who have taught and helped me during the past two years at The Ohio State University, particularly my advisor, Dr.