PRODUCT MONOGRAPH Pr WELLBUTRIN XL Bupropion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WELLBUTRIN SR Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION psychosis, hallucinations, paranoia, delusions, homicidal ideation, These highlights do not include all the information needed to use aggression, hostility, agitation, anxiety, and panic, as well as suicidal WELLBUTRIN SR safely and effectively. See full prescribing ideation, suicide attempt, and completed suicide. Observe patients information for WELLBUTRIN SR. attempting to quit smoking with bupropion for the occurrence of such symptoms and instruct them to discontinue bupropion and contact a WELLBUTRIN SR (bupropion hydrochloride) sustained-release tablets, healthcare provider if they experience such adverse events. (5.2) for oral use • Initial U.S. Approval: 1985 Seizure risk: The risk is dose-related. Can minimize risk by gradually increasing the dose and limiting daily dose to 400 mg. Discontinue if WARNING: SUICIDAL THOUGHTS AND BEHAVIORS seizure occurs. (4, 5.3, 7.3) See full prescribing information for complete boxed warning. • Hypertension: WELLBUTRIN SR can increase blood pressure. Monitor blood pressure before initiating treatment and periodically during • Increased risk of suicidal thinking and behavior in children, treatment. (5.4) adolescents and young adults taking antidepressants. (5.1) • Activation of mania/hypomania: Screen patients for bipolar disorder and • Monitor for worsening and emergence of suicidal thoughts and monitor for these symptoms. (5.5) behaviors. (5.1) • Psychosis and other neuropsychiatric reactions: Instruct patients to contact a healthcare professional if such reactions occur. (5.6) --------------------------- INDICATIONS AND USAGE ---------------------------- • Angle-closure glaucoma: Angle-closure glaucoma has occurred in WELLBUTRIN SR is an aminoketone antidepressant, indicated for the patients with untreated anatomically narrow angles treated with treatment of major depressive disorder (MDD). (1) antidepressants. -

Clarifying the Ghrelin System's Ability to Regulate Feeding Behaviours
International Journal of Molecular Sciences Review Clarifying the Ghrelin System’s Ability to Regulate Feeding Behaviours Despite Enigmatic Spatial Separation of the GHSR and Its Endogenous Ligand Alexander Edwards and Alfonso Abizaid * Department of Neuroscience, Carleton University, 1125 Colonel By Drive, Ottawa, ON K1S 5B6, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-613-520-2600 (ext. 1544) Academic Editor: Suzanne L. Dickson Received: 27 February 2017; Accepted: 11 April 2017; Published: 19 April 2017 Abstract: Ghrelin is a hormone predominantly produced in and secreted from the stomach. Ghrelin is involved in many physiological processes including feeding, the stress response, and in modulating learning, memory and motivational processes. Ghrelin does this by binding to its receptor, the growth hormone secretagogue receptor (GHSR), a receptor found in relatively high concentrations in hypothalamic and mesolimbic brain regions. While the feeding and metabolic effects of ghrelin can be explained by the effects of this hormone on regions of the brain that have a more permeable blood brain barrier (BBB), ghrelin produced within the periphery demonstrates a limited ability to reach extrahypothalamic regions where GHSRs are expressed. Therefore, one of the most pressing unanswered questions plaguing ghrelin research is how GHSRs, distributed in brain regions protected by the BBB, are activated despite ghrelin’s predominant peripheral production and poor ability to transverse the BBB. This manuscript will describe how peripheral ghrelin activates central GHSRs to encourage feeding, and how central ghrelin synthesis and ghrelin independent activation of GHSRs may also contribute to the modulation of feeding behaviours. Keywords: feeding; ghrelin; GHSR; blood brain barrier; vagal afferents; circumventricular organs; central ghrelin synthesis; GHSR heterodimerization; GHSR constitutive activity 1. -

WHO Drug Information Vol. 12, No. 3, 1998
WHO DRUG INFORMATION VOLUME 12 NUMBER 3 • 1998 RECOMMENDED INN LIST 40 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA Volume 12, Number 3, 1998 World Health Organization, Geneva WHO Drug Information Contents Seratrodast and hepatic dysfunction 146 Meloxicam safety similar to other NSAIDs 147 Proxibarbal withdrawn from the market 147 General Policy Issues Cholestin an unapproved drug 147 Vigabatrin and visual defects 147 Starting materials for pharmaceutical products: safety concerns 129 Glycerol contaminated with diethylene glycol 129 ATC/DDD Classification (final) 148 Pharmaceutical excipients: certificates of analysis and vendor qualification 130 ATC/DDD Classification Quality assurance and supply of starting (temporary) 150 materials 132 Implementation of vendor certification 134 Control and safe trade in starting materials Essential Drugs for pharmaceuticals: recommendations 134 WHO Model Formulary: Immunosuppressives, antineoplastics and drugs used in palliative care Reports on Individual Drugs Immunosuppresive drugs 153 Tamoxifen in the prevention and treatment Azathioprine 153 of breast cancer 136 Ciclosporin 154 Selective serotonin re-uptake inhibitors and Cytotoxic drugs 154 withdrawal reactions 136 Asparaginase 157 Triclabendazole and fascioliasis 138 Bleomycin 157 Calcium folinate 157 Chlormethine 158 Current Topics Cisplatin 158 Reverse transcriptase activity in vaccines 140 Cyclophosphamide 158 Consumer protection and herbal remedies 141 Cytarabine 159 Indiscriminate antibiotic -

The Pharmacology of Amiodarone and Digoxin As Antiarrhythmic Agents
Part I Anaesthesia Refresher Course – 2017 University of Cape Town The Pharmacology of Amiodarone and Digoxin as Antiarrhythmic Agents Dr Adri Vorster UCT Department of Anaesthesia & Perioperative Medicine The heart contains pacemaker, conduction and contractile tissue. Cardiac arrhythmias are caused by either enhancement or depression of cardiac action potential generation by pacemaker cells, or by abnormal conduction of the action potential. The pharmacological treatment of arrhythmias aims to achieve restoration of a normal rhythm and rate. The resting membrane potential of myocytes is around -90 mV, with the inside of the membrane more negative than the outside. The main extracellular ions are Na+ and Cl−, with K+ the main intracellular ion. The cardiac action potential involves a change in voltage across the cell membrane of myocytes, caused by movement of charged ions across the membrane. This voltage change is triggered by pacemaker cells. The action potential is divided into 5 phases (figure 1). Phase 0: Rapid depolarisation Duration < 2ms Threshold potential must be reached (-70 mV) for propagation to occur Rapid positive charge achieved as a result of increased Na+ conductance through voltage-gated Na+ channels in the cell membrane Phase 1: Partial repolarisation Closure of Na+ channels K+ channels open and close, resulting in brief outflow of K+ and a more negative membrane potential Phase 2: Plateau Duration up to 150 ms Absolute refractory period – prevents further depolarisation and myocardial tetany Result of Ca++ influx -

Dabigatran Amoxicillin Clavulanate IV Treatment in the Community
BEST PRACTICE 38 SEPTEMBER 2011 Dabigatran Amoxicillin clavulanate bpac nz IV treatment in the community better medicin e Editor-in-chief We would like to acknowledge the following people for Professor Murray Tilyard their guidance and expertise in developing this edition: Professor Carl Burgess, Wellington Editor Dr Gerry Devlin, Hamilton Rebecca Harris Dr John Fink, Christchurch Dr Lisa Houghton, Dunedin Programme Development Dr Rosemary Ikram, Christchurch Mark Caswell Dr Sisira Jayathissa, Wellington Rachael Clarke Kate Laidlow, Rotorua Peter Ellison Dr Hywel Lloyd, GP Reviewer, Dunedin Julie Knight Associate Professor Stewart Mann, Wellington Noni Richards Dr Richard Medlicott, Wellington Dr AnneMarie Tangney Dr Alan Panting, Nelson Dr Sharyn Willis Dr Helen Patterson, Dunedin Dave Woods David Rankin, Wellington Report Development Dr Ralph Stewart, Auckland Justine Broadley Dr Neil Whittaker, GP Reviewer, Nelson Tim Powell Dr Howard Wilson, Akaroa Design Michael Crawford Best Practice Journal (BPJ) ISSN 1177-5645 Web BPJ, Issue 38, September 2011 Gordon Smith BPJ is published and owned by bpacnz Ltd Management and Administration Level 8, 10 George Street, Dunedin, New Zealand. Jaala Baldwin Bpacnz Ltd is an independent organisation that promotes health Kaye Baldwin care interventions which meet patients’ needs and are evidence Tony Fraser based, cost effective and suitable for the New Zealand context. Kyla Letman We develop and distribute evidence based resources which describe, facilitate and help overcome the barriers to best Clinical Advisory Group practice. Clive Cannons nz Michele Cray Bpac Ltd is currently funded through contracts with PHARMAC and DHBNZ. Margaret Gibbs nz Dr Rosemary Ikram Bpac Ltd has five shareholders: Procare Health, South Link Health, General Practice NZ, the University of Otago and Pegasus Dr Cam Kyle Health. -

FSI-D-16-00226R1 Title
Elsevier Editorial System(tm) for Forensic Science International Manuscript Draft Manuscript Number: FSI-D-16-00226R1 Title: An overview of Emerging and New Psychoactive Substances in the United Kingdom Article Type: Review Article Keywords: New Psychoactive Substances Psychostimulants Lefetamine Hallucinogens LSD Derivatives Benzodiazepines Corresponding Author: Prof. Simon Gibbons, Corresponding Author's Institution: UCL School of Pharmacy First Author: Simon Gibbons Order of Authors: Simon Gibbons; Shruti Beharry Abstract: The purpose of this review is to identify emerging or new psychoactive substances (NPS) by undertaking an online survey of the UK NPS market and to gather any data from online drug fora and published literature. Drugs from four main classes of NPS were identified: psychostimulants, dissociative anaesthetics, hallucinogens (phenylalkylamine-based and lysergamide-based materials) and finally benzodiazepines. For inclusion in the review the 'user reviews' on drugs fora were selected based on whether or not the particular NPS of interest was used alone or in combination. NPS that were use alone were considered. Each of the classes contained drugs that are modelled on existing illegal materials and are now covered by the UK New Psychoactive Substances Bill in 2016. Suggested Reviewers: Title Page (with authors and addresses) An overview of Emerging and New Psychoactive Substances in the United Kingdom Shruti Beharry and Simon Gibbons1 Research Department of Pharmaceutical and Biological Chemistry UCL School of Pharmacy -

Full Prescribing Information Warning: Suicidal Thoughts
tablets (SR) BUPROPION hydrochloride extended-release HIGHLIGHTS OF PRESCRIBING INFORMATION anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Observe hydrochloride extended-release tablets (SR) was reported. However, the symptoms persisted in some Table 3. Adverse Reactions Reported by at Least 1% of Subjects on Active Treatment and at a tablets (SR) may be necessary when coadministered with ritonavir, lopinavir, or efavirenz [see Clinical These highlights do not include all the information needed to use bupropion hydrochloride patients attempting to quit smoking with Bupropion hydrochloride extended-release tablets, USP cases; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve. Greater Frequency than Placebo in the Comparator Trial Pharmacology (12.3)] but should not exceed the maximum recommended dose. extended-release tablets (SR) safely and effectively. See full prescribing information for (SR) for the occurrence of such symptoms and instruct them to discontinue Bupropion hydrochloride Carbamazepine, Phenobarbital, Phenytoin: While not systematically studied, these drugs extended-release tablets, USP (SR) and contact a healthcare provider if they experience such adverse The neuropsychiatric safety of Bupropion hydrochloride extended-release tablets (SR) was evaluated in Bupropion Bupropion may induce the metabolism of bupropion and may decrease bupropion exposure [see Clinical bupropion hydrochloride extended-release tablets (SR). Nicotine events. (5.2) a randomized, double-blind, active-and placebo-controlled study that included patients without a history Hydrochloride Hydrochloride Pharmacology (12.3)]. If bupropion is used concomitantly with a CYP inducer, it may be necessary Transdermal BUPROPION hydrochloride extended-release tablets (SR), for oral use • Seizure risk: The risk is dose-related. -

Spectrum of Digoxin-Induced Ocular Toxicity: a Case Report and Literature Review Delphine Renard1*, Eve Rubli2, Nathalie Voide3, François‑Xavier Borruat3 and Laura E
Renard et al. BMC Res Notes (2015) 8:368 DOI 10.1186/s13104-015-1367-6 CASE REPORT Open Access Spectrum of digoxin-induced ocular toxicity: a case report and literature review Delphine Renard1*, Eve Rubli2, Nathalie Voide3, François‑Xavier Borruat3 and Laura E. Rothuizen1 Abstract Background: Digoxin intoxication results in predominantly digestive, cardiac and neurological symptoms. This case is outstanding in that the intoxication occurred in a nonagenarian and induced severe, extensively documented visual symptoms as well as dysphagia and proprioceptive illusions. Moreover, it went undiagnosed for a whole month despite close medical follow-up, illustrating the difficulty in recognizing drug-induced effects in a polymorbid patient. Case presentation: Digoxin 0.25 mg qd for atrial fibrillation was prescribed to a 91-year-old woman with an esti‑ mated creatinine clearance of 18 ml/min. Over the following 2–3 weeks she developed nausea, vomiting and dyspha‑ gia, snowy and blurry vision, photopsia, dyschromatopsia, aggravated pre-existing formed visual hallucinations and proprioceptive illusions. She saw her family doctor twice and visited the eye clinic once until, 1 month after starting digoxin, she was admitted to the emergency room. Intoxication was confirmed by a serum digoxin level of 5.7 ng/ml (reference range 0.8–2 ng/ml). After stopping digoxin, general symptoms resolved in a few days, but visual complaints persisted. Examination by the ophthalmologist revealed decreased visual acuity in both eyes, 4/10 in the right eye (OD) and 5/10 in the left eye (OS), decreased color vision as demonstrated by a score of 1/13 in both eyes (OU) on Ishihara pseudoisochromatic plates, OS cataract, and dry age-related macular degeneration (ARMD). -

Cross-Sectional Study of the Dispensation of Synthetic Anorectic Drugs in Community Pharmacies in the City of Cruz Alta – State of Rio Grande Do Sul
Brazilian Journal of Pharmaceutical Sciences vol. 50, n. 4, oct./dec., 2014 Article http://dx.doi.org/10.1590/S1984-82502014000400008 Cross-sectional study of the dispensation of synthetic anorectic drugs in community pharmacies in the city of Cruz Alta – State of Rio Grande do Sul Marcieli Maria Navarini1, Viviane Cecilia Kessler Nunes Deuschle2,*, Regis Augusto Norbert Deuschle3 1Faculty of Pharmacy, University of Cruz Alta, Rio Grande do Sul, Brazil,2,3Postgraduate Program in Pharmaceutical Sciences, Federal University of Santa Maria, Brazil Obesity is defined as the excess adipose tissue in the body. Drugs responsible for inhibiting the appetite are called anorectics or appetite suppressants. Sibutramine, fenproporex and amfepramone belongs to this class, and are capable of causing physical or psychological dependence. The aim of this study was to evaluate the frequency of prescriptions for appetite suppressants in community pharmacies at Cruz Alta, State of Rio Grande do Sul, Brazil. The sales of fenproporex, amfepramone and sibutramine in the months of September, October and November 2010 and April, May and June 2011 were compared. It was observed that the most commonly dispensed anorectic in the three community pharmacies analyzed was sibutramine. In the months of September, October and November 2010, consumption was higher, with sibutramine achieving 40.3% of overall sales, amfepramone 21% and, finally, fenproporex, 7.9%. The consumption of appetite suppressants was more prevalent in females, who represented 82% of total. The results suggested the existence of high consumption of anorectics, possibly related to the current concern with aesthetic standards, which emphasizes the importance of strict control over the marketing of these substances. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
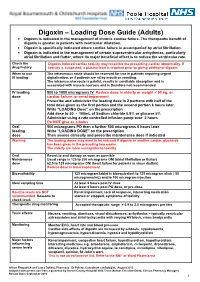
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -

The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats
Neuropsychopharmacology (2017) 42, 2292–2300 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats 1 1 ,1 Claire J Foldi , Laura K Milton and Brian J Oldfield* 1 Department of Physiology, Monash University, Clayton, VIC, Australia Patients suffering from anorexia nervosa (AN) become anhedonic; unable or unwilling to derive normal pleasures and avoid rewarding outcomes, most profoundly in food intake. The activity-based anorexia (ABA) model recapitulates many of the characteristics of the human condition, including anhedonia, and allows investigation of the underlying neurobiology of AN. The potential for increased neuronal activity in reward/hedonic circuits to prevent and rescue weight loss is investigated in this model. The mesolimbic pathway extending from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) was activated using a dual viral strategy, involving retrograde transport of Cre (CAV-2-Cre) to the VTA and coincident injection of DREADD receptors (AAV-hSyn-DIO-hM3D(Gq)-mCherry). Systemic clozapine-n-oxide (CNO; 0.3 mg/kg) successfully recruited a large proportion of the VTA-NAc dopaminergic projections, with activity evidenced by colocalization with elevated levels of Fos protein. The effects of reward circuit activation on energy balance and predicted survival was investigated in female Sprague-Dawley rats, where free access to running wheels was paired with time-limited (90 min) access to food, a paradigm (ABA) which will cause anorexia and death if unchecked. Excitation of the reward pathway substantially increased food intake and food anticipatory activity (FAA) to prevent ABA-associated weight loss, while overall locomotor activity was unchanged.