The Pharmacology of Amiodarone and Digoxin As Antiarrhythmic Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dabigatran Amoxicillin Clavulanate IV Treatment in the Community
BEST PRACTICE 38 SEPTEMBER 2011 Dabigatran Amoxicillin clavulanate bpac nz IV treatment in the community better medicin e Editor-in-chief We would like to acknowledge the following people for Professor Murray Tilyard their guidance and expertise in developing this edition: Professor Carl Burgess, Wellington Editor Dr Gerry Devlin, Hamilton Rebecca Harris Dr John Fink, Christchurch Dr Lisa Houghton, Dunedin Programme Development Dr Rosemary Ikram, Christchurch Mark Caswell Dr Sisira Jayathissa, Wellington Rachael Clarke Kate Laidlow, Rotorua Peter Ellison Dr Hywel Lloyd, GP Reviewer, Dunedin Julie Knight Associate Professor Stewart Mann, Wellington Noni Richards Dr Richard Medlicott, Wellington Dr AnneMarie Tangney Dr Alan Panting, Nelson Dr Sharyn Willis Dr Helen Patterson, Dunedin Dave Woods David Rankin, Wellington Report Development Dr Ralph Stewart, Auckland Justine Broadley Dr Neil Whittaker, GP Reviewer, Nelson Tim Powell Dr Howard Wilson, Akaroa Design Michael Crawford Best Practice Journal (BPJ) ISSN 1177-5645 Web BPJ, Issue 38, September 2011 Gordon Smith BPJ is published and owned by bpacnz Ltd Management and Administration Level 8, 10 George Street, Dunedin, New Zealand. Jaala Baldwin Bpacnz Ltd is an independent organisation that promotes health Kaye Baldwin care interventions which meet patients’ needs and are evidence Tony Fraser based, cost effective and suitable for the New Zealand context. Kyla Letman We develop and distribute evidence based resources which describe, facilitate and help overcome the barriers to best Clinical Advisory Group practice. Clive Cannons nz Michele Cray Bpac Ltd is currently funded through contracts with PHARMAC and DHBNZ. Margaret Gibbs nz Dr Rosemary Ikram Bpac Ltd has five shareholders: Procare Health, South Link Health, General Practice NZ, the University of Otago and Pegasus Dr Cam Kyle Health. -

Tricyclic Antidepressant
Princess Margaret Hospital for Children Emergency Department Guideline PAEDIATRIC ACUTE CARE GUIDELINE Poisoning – Tricyclic Antidepressant Scope (Staff): All Emergency Department Clinicians Scope (Area): Emergency Department This document should be read in conjunction with this DISCLAIMER http://kidshealthwa.com/about/disclaimer/ Poisoning – Tricyclic Antidepressant This guideline is a general approach to tricyclic antidepressant poisoning. For specific details please contact Poisons Information: 131126 or refer to the Toxicology Handbook. Agents: Amitriptyline Clomipramine Dothiepin Doxepin Imipramine Nortriptyline Trimipramine Background Tricyclic antidepressants (TCAs) act on a variety of receptors whose actions include: Noradrenaline reuptake inhibition Central and peripheral anticholinergic effect Fast sodium channel blockade in the myocardium Peripheral alpha1-adrenergic receptor blockade The life threatening effects of acute tricyclic antidepressant (TCA) overdose are: Rapid onset of coma Seizures Cardiac dysrhythmias Page 1 of 6 Emergency Department Guideline Poisoning – Tricyclic Antidepressant Hypotension and central and peripheral anticholinergic effects may also be seen Risk Assessment Most acute accidental paediatric exposures do not result in life threatening toxicity A 10kg child can develop life threatening poisoning with the ingestion of a single tablet (e.g. 150mg amitriptyline) Patients who ingest a large dose of TCA usually develop evidence of intoxication within 2-4 hours, and always within 6 hours If their is suspicion -

Full Prescribing Information Warning: Suicidal Thoughts
tablets (SR) BUPROPION hydrochloride extended-release HIGHLIGHTS OF PRESCRIBING INFORMATION anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Observe hydrochloride extended-release tablets (SR) was reported. However, the symptoms persisted in some Table 3. Adverse Reactions Reported by at Least 1% of Subjects on Active Treatment and at a tablets (SR) may be necessary when coadministered with ritonavir, lopinavir, or efavirenz [see Clinical These highlights do not include all the information needed to use bupropion hydrochloride patients attempting to quit smoking with Bupropion hydrochloride extended-release tablets, USP cases; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve. Greater Frequency than Placebo in the Comparator Trial Pharmacology (12.3)] but should not exceed the maximum recommended dose. extended-release tablets (SR) safely and effectively. See full prescribing information for (SR) for the occurrence of such symptoms and instruct them to discontinue Bupropion hydrochloride Carbamazepine, Phenobarbital, Phenytoin: While not systematically studied, these drugs extended-release tablets, USP (SR) and contact a healthcare provider if they experience such adverse The neuropsychiatric safety of Bupropion hydrochloride extended-release tablets (SR) was evaluated in Bupropion Bupropion may induce the metabolism of bupropion and may decrease bupropion exposure [see Clinical bupropion hydrochloride extended-release tablets (SR). Nicotine events. (5.2) a randomized, double-blind, active-and placebo-controlled study that included patients without a history Hydrochloride Hydrochloride Pharmacology (12.3)]. If bupropion is used concomitantly with a CYP inducer, it may be necessary Transdermal BUPROPION hydrochloride extended-release tablets (SR), for oral use • Seizure risk: The risk is dose-related. -

Spectrum of Digoxin-Induced Ocular Toxicity: a Case Report and Literature Review Delphine Renard1*, Eve Rubli2, Nathalie Voide3, François‑Xavier Borruat3 and Laura E
Renard et al. BMC Res Notes (2015) 8:368 DOI 10.1186/s13104-015-1367-6 CASE REPORT Open Access Spectrum of digoxin-induced ocular toxicity: a case report and literature review Delphine Renard1*, Eve Rubli2, Nathalie Voide3, François‑Xavier Borruat3 and Laura E. Rothuizen1 Abstract Background: Digoxin intoxication results in predominantly digestive, cardiac and neurological symptoms. This case is outstanding in that the intoxication occurred in a nonagenarian and induced severe, extensively documented visual symptoms as well as dysphagia and proprioceptive illusions. Moreover, it went undiagnosed for a whole month despite close medical follow-up, illustrating the difficulty in recognizing drug-induced effects in a polymorbid patient. Case presentation: Digoxin 0.25 mg qd for atrial fibrillation was prescribed to a 91-year-old woman with an esti‑ mated creatinine clearance of 18 ml/min. Over the following 2–3 weeks she developed nausea, vomiting and dyspha‑ gia, snowy and blurry vision, photopsia, dyschromatopsia, aggravated pre-existing formed visual hallucinations and proprioceptive illusions. She saw her family doctor twice and visited the eye clinic once until, 1 month after starting digoxin, she was admitted to the emergency room. Intoxication was confirmed by a serum digoxin level of 5.7 ng/ml (reference range 0.8–2 ng/ml). After stopping digoxin, general symptoms resolved in a few days, but visual complaints persisted. Examination by the ophthalmologist revealed decreased visual acuity in both eyes, 4/10 in the right eye (OD) and 5/10 in the left eye (OS), decreased color vision as demonstrated by a score of 1/13 in both eyes (OU) on Ishihara pseudoisochromatic plates, OS cataract, and dry age-related macular degeneration (ARMD). -

Amiodarone (As Hydrochloride)
NEW ZEALAND DATA SHEET ARATAC 1. Product Name Aratac, 100 mg and 200 mg tablet. 2. Qualitative and Quantitative Composition Each Aratac tablet contains 100 mg or 200 mg of amiodarone (as hydrochloride). Aratac tablets contain lactose. For the full list of excipients, see section 6.1. Amiodarone hydrochloride is a fine white crystalline powder. It is slightly soluble in water and is soluble in alcohol and chloroform. It is an amphiphilic compound and contains iodine in its formulation. Each 200 mg tablet of amiodarone contains approximately 75 mg organic iodine. In the steady state, metabolism of 300 mg amiodarone yields 9 mg/day of iodine. 3. Pharmaceutical Form Amiodarone 100 mg Tablet: round, normal convex, white tablet, 8.5 mm diameter, imprinted “AM” | “100” on one side and “G” on the other. Amiodarone 200 mg Tablet: round, normal convex, white tablet, 10.0 mm diameter, imprinted “AM” | “200” on one side and “G” on the other. The tablet can be divided into equal doses. 4. Clinical Particulars 4.1 Therapeutic indications Treatment should be initiated only under hospital or specialist supervision. Tachyarrhythmias associated with Wolff-Parkinson-White Syndrome. Atrial flutter and fibrillation when other agents cannot be used. All types of tachyarrhythmias of paroxysmal nature including: supraventricular, nodal and ventricular tachycardias, ventricular fibrillation; when other agents cannot be used. Tablets are used for stabilisation and long term treatment. 4.2 Dose and method of administration Dose Due to poor absorption and wide inter-patient variability of absorption, the initial loading and subsequent maintenance dosage schedules of the medicine in clinical use has to be individually titrated. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
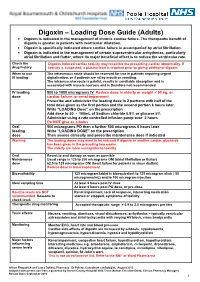
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -

PRODUCT MONOGRAPH Pr WELLBUTRIN XL Bupropion
PRODUCT MONOGRAPH Pr WELLBUTRIN XL Bupropion Hydrochloride Extended-Release Tablets, USP 150 mg and 300 mg Antidepressant Name: Date of Revision: Valeant Canada LP March 20, 2017 Address: 2150 St-Elzear Blvd. West Laval, QC, H7L 4A8 Control Number: 200959 Table of Contents Page PART I: HEALTH PROFESSIONAL INFORMATION .............................................................. 2 SUMMARY PRODUCT INFORMATION ................................................................................. 2 INDICATIONS AND CLINICAL USE ....................................................................................... 2 CONTRAINDICATIONS ............................................................................................................ 3 WARNINGS AND PRECAUTIONS ........................................................................................... 3 ADVERSE REACTIONS ............................................................................................................. 9 DRUG INTERACTIONS ........................................................................................................... 19 DOSAGE AND ADMINISTRATION ....................................................................................... 22 OVERDOSAGE ......................................................................................................................... 24 ACTION AND CLINICAL PHARMACOLOGY ..................................................................... 25 STORAGE AND STABILITY .................................................................................................. -

Treatment for Calcium Channel Blocker Poisoning: a Systematic Review
Clinical Toxicology (2014), 52, 926–944 Copyright © 2014 Informa Healthcare USA, Inc. ISSN: 1556-3650 print / 1556-9519 online DOI: 10.3109/15563650.2014.965827 REVIEW ARTICLE Treatment for calcium channel blocker poisoning: A systematic review M. ST-ONGE , 1,2,3 P.-A. DUB É , 4,5,6 S. GOSSELIN ,7,8,9 C. GUIMONT , 10 J. GODWIN , 1,3 P. M. ARCHAMBAULT , 11,12,13,14 J.-M. CHAUNY , 15,16 A. J. FRENETTE , 15,17 M. DARVEAU , 18 N. LE SAGE , 10,14 J. POITRAS , 11,12 J. PROVENCHER , 19 D. N. JUURLINK , 1,20,21 and R. BLAIS 7 1 Ontario and Manitoba Poison Centre, Toronto, ON, Canada 2 Institute of Medical Science, University of Toronto, Toronto, ON, Canada 3 Department of Clinical Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada 4 Direction of Environmental Health and Toxicology, Institut national de sant é publique du Qu é bec, Qu é bec, QC, Canada 5 Centre Hospitalier Universitaire de Qu é bec, Qu é bec, QC, Canada 6 Faculty of Pharmacy, Université Laval, Qu é bec, QC, Canada 7 Centre antipoison du Qu é bec, Qu é bec, QC, Canada 8 Department of Medicine, McGill University, Montr é al, QC, Canada 9 Toxicology Consulting Service, McGill University Health Centre, Montr é al, QC, Canada 10 Centre Hospitalier Universitaire de Qu é bec, Qu é bec, QC, Canada 11 Centre de sant é et services sociaux Alphonse-Desjardins (CHAU de Lévis), L é vis, QC, Canada 12 Department of Family Medicine and Emergency Medicine, Universit é Laval, Québec, QC, Canada 13 Division de soins intensifs, Universit é Laval, Qu é bec, QC, Canada 14 Populations -

Guidelines for Use of Digoxin (Lanoxin )
Guidelines for use of Digoxin (Lanoxin) Recommended Neonatal Dose, Route, and Interval Loading or digitalizing dose: Total Loading Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) 29 15 20 30 to 36 20 25 37 to 48 30 40 49 40 50 Divide into 3 doses over 24 hours Generally given in the following manner: 1. One-half the loading dose given immediately IV or PO 2. One-fourth the loading dose given 8 to 12 hours later IV or PO 3. The remaining one-fourth loading dose given after an additional 8 to 12 hours IV or PO 4. Administer IV slow push over 5 to 10 minutes 5. Obtain ECG 6 hours after digitalizing dose to assess for toxicity Maintenance dose: should be started 12 hours after the loading dose is completed Maintenance Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) Interval (hours) 29 4 5 24 30 to 36 5 6 24 37 to 48 4 5 12 49 5 6 12 Titrate based on clinical response Chief Indications 1. Heart failure caused by diminished myocardial contractility 2. Supraventricular tachycardia, atrial flutter, atrial fibrillation Possible Adverse Reactions 1. Atrial or ventricular arrhythmias are common and may be an early indication of overdosage 2. Feeding intolerance, vomiting, diarrhea 3. Hypokalemia is associated with chronic Digoxin toxicity 4. Bradycardia due to depression of A-V conduction 5. Diuresis from improved cardiac output 6. Lethargy or seizures with toxicity Contraindications & Precautions 1. CAUTION use with pre-existing hypokalemia may lead to adverse reactions 2. CAUTION use with Indomethacin - may inhibit excretion of Digoxin 3. -

Amiodarone Enhances Anticonvulsive Effect of Oxcarbazepine and Pregabalin in the Mouse Maximal Electroshock Model
International Journal of Molecular Sciences Article Amiodarone Enhances Anticonvulsive Effect of Oxcarbazepine and Pregabalin in the Mouse Maximal Electroshock Model Monika Banach 1 , Monika Rudkowska 1, Agata Sumara 2 and Kinga Borowicz-Reutt 1,* 1 Independent Unit of Experimental Neuropathophysiology, Medical University of Lublin, Jaczewskiego 8b, PL-20-090 Lublin, Poland; [email protected] (M.B.); [email protected] (M.R.) 2 Department of Pathophysiology, Medical University of Lublin, Jaczewskiego 8b, PL-20-090 Lublin, Poland; [email protected] * Correspondence: [email protected] Abstract: Accumulating experimental studies show that antiarrhythmic and antiepileptic drugs share some molecular mechanisms of action and can interact with each other. In this study, the influence of amiodarone (a class III antiarrhythmic drug) on the antiseizure action of four second-generation antiepileptic drugs was evaluated in the maximal electroshock model in mice. Amiodarone, although ineffective in the electroconvulsive threshold test, significantly potentiated the antielectroshock activ- ity of oxcarbazepine and pregabalin. Amiodarone, given alone or in combination with oxcarbazepine, lamotrigine, or topiramate, significantly disturbed long-term memory in the passive-avoidance task in mice. Brain concentrations of antiepileptic drugs were not affected by amiodarone. However, the brain concentration of amiodarone was significantly elevated by oxcarbazepine, topiramate, and pregabalin. Additionally, oxcarbazepine and pregabalin elevated the brain concentration of desethylamiodarone, the main metabolite of amiodarone. In conclusion, potentially beneficial action of amiodarone in epilepsy patients seems to be limited by neurotoxic effects of amiodarone. Although results of this study should still be confirmed in chronic protocols of treatment, special precautions Citation: Banach, M.; Rudkowska, are recommended in clinical conditions. -

Xenical, INN-Orlistat
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Xenical. This scientific discussion has been updated until 1 November 2003. For information on changes after this date please refer to module 8B. 1. Introduction Obesity is a disease characterised by an excess body fat. It is often measured by calculation of the body mass index (BMI), i.e. the body weight in kg divided by body surface area in m2. Individuals may be regarded as obese with a BMI >25-27 kg/m² (depending on age). A number of concomitant pathological processes and diseases are associated with obesity including coronary heart disease, hypertension, stroke, non-insulin dependent diabetes mellitus and certain forms of cancer. Besides changes in diet, behaviour and physical activities, obesity may be treated by surgery or pharmacological therapy. All currently available medicinal products for obesity treatment are appetite suppressants (amphetamine-like products) that act via the central nervous system (CNS). However, they may not be prescribed during longer periods than 3 months, due to the potential risk of abuse. Thus, there is a need for medicinal products that can be used in chronic treatment together with dietary and behavioural modifications. Orlistat belongs to a new class of pharmacological agents. It inhibits the action of gastrointestinal lipases and thereby impairs the metabolism of lipids in the intestinal lumen leading to a prevention of lipid absorption. Xenical is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI) greater or equal to 30 kg/m², or overweight patients (BMI > 28 kg/m²) with associated risk factors.