XA9847628 Chapter 31 KIDNEYS and URINARY SYSTEM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detection of Continuous Erythropoietin Receptor Activator in Blood And
Letters to the Editor 5 A Pugliese-Ciaccio, Catanzaro; Dipartimento di Oncologia, 1.0 Biologia e Genetica Università degli Studi di Genova, Italy. Funding: supported from Associazione Italiana Ricerca sul Cancro 0.8 (AIRC) (to FM and MF) and Fondazione ‘Amelia Scorza’ β2-mneg Onlus, Cosenza, Italy. 0.6 Acknowledgments: we would like to acknowledge Dr. Vincenzo not treated Callea, Prof Luca Baldini, Dr Ugo Consoli and Dr Serena Matis 0.4 for their contribution and useful suggestions.We thank Laura Veroni and Brigida Gulino for precious secretarial assistance. Proportion 0.2 p=0.002 pos β β2-m Key words: 2-microglobulin, CD38, IgVH mutational status, CLL, prognosis. 0.0 Correspondence: Fortunato Morabito, Unità Operativa Complessa di Ematologia, Dipartimento di Medicina Interna, 0 3 6 9 12 15 Azienda Ospedaliera di Cosenza, Viale della Repubblica, years 87100 Cosenza, Italy. Phone: international +39.0984.681329. B Fax: international +39.0984.791751. Univariate analysis E-mail: [email protected] Risk categories HR (95% C.I., p value) Citation: Gentile M, Cutrona G, Neri A, Molica S, Ferrarini M, No factor 1 and Morabito F. Predictive value of B2-microglobulin (B2-m) levels One factor 1,5 (0.7-3.4, p=ns) in chronic lymphocytic leukemia since Binet A stages. Two factor 5.0 (2.5-10.2, p<0.0001) Haematologica 2009; 94:887-888. Three factors 15.4 (7.3-32.5, p<0.0001) doi:10.3324/haematol.2009.005561 C 1.0 References 0.8 1. Rossi D, Zucchetto A, Rossi FM, Capello D, Cerri M, no factor Deambrogi C, et al. -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

St. Lawrence School Subject
St. Lawrence School Subject - Science Class - 4 Chapter - 3 Human Body : Digestive and Excetory System ( Part - 1 ) Learn about * Digestive system * Excretory system * Healthy eating habits Digestive System The process by which food is broken down into a simpler form so that it can be easily taken in or absorbed by our body is called digestion. Many organs work together and help in the process of digestion. The mouth, food pipe, stomach, small and large intestine, liver, rectum, and anus are the main organs of the digestive system. Let us learn about them. Mouth Digestion starts in the mouth. The teeth help to break down and chew food. The chewed food then mixes with a liquid, called saliva, produced in our mouth. It makes the food softer and easier to swallow. The tongue helps in the proper mixing of saliva with the food. Food pipe The food pipe ( oesophagus ) passes the food from the mouth to the stomach. Stomach Inside the stomach, the food is broken down further into smaller pieces by churning and with the help of chemicals called digestive juices. Small intestine From the small intestine, the undigested food passes into the large intestine. The large intestine is a shorter but wider, tube - like structure, which collects the indigestible food from the small intestine. The large intestine absorbs water from this undigested food and forms waste products called faeces. Rectum Rectum is the final part of the large intestine. Faeces are stored in the rectum for a short time before being passed out through anus. Anus Faeces are removed from the body through the anus. -

Urinary Bladder – Proteinaceous Plug
Urinary bladder – Proteinaceous Plug Figure Legend: Figure 1 An eosinophilic amorphous proteinaceous plug in the bladder lumen from a male B6C3F1 mouse in a chronic study. Figure 2 A proteinaceous plug associated with other flocculent, eosinophilic material, from a male F344/N rat in an acute study. Comment: Proteinaceous plugs are commonly noted as a postmortem change resulting from an agonal secretion of accessory sex gland fluids during euthanasia. Proteinaceous plugs vary in size but can be large, filling the urinary bladder (Figure 1 and Figure 2). Microscopically, the plug is composed of a mixture of an amorphous eosinophilic material, sometimes containing desquamated epithelial cells and spermatozoa. Proteinaceous plugs by themselves have no toxicologic importance and are not precursors of calculi. Plugs may be seen with obstructive syndromes associated with bacterial inflammation. They must be differentiated from calculi. Recommendation: Proteinaceous plugs occurring alone and not associated with any pathologic lesions should be recognized as an artifact and should not be diagnosed. Occasionally, proteinaceous plugs are recognized grossly, and the pathologist should use his or her judgment to correlate the gross lesion to an artifactual proteinaceous plug. 1 Urinary bladder – Proteinaceous Plug References: Gaillard ET. 1999. Ureter, urinary bladder and urethra. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 235– 258. Abstract: http://www.cacheriverpress.com/books/pathmouse.htm Hard GC, Alden CL, Bruner RH, Frith CH, Lewis RM, Owen RA, Krieg K, Durchfeld-Meyer B. 1999. Non-proliferative lesions of the kidney and lower urinary tract in rats. -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

1535190852 9 Medical Terminology Urinary.Pdf
Syrian Private University Medical Faculty Medical Terminology M.A.Kubtan , MD – FRCS Lecture 9 M.A.Kubtan Objectives After studying this chapter, you will be able to: •Name the parts of the urinary system and discuss the function of each part •Define combining forms used in building words that relate to the urinary system •Identify the meaning of related abbreviations •Name the common diagnoses, clinical procedures, and laboratory tests used in treating disorders of the urinary system M.A.Kubtan 2 Objectives Part 2 •List and define the major pathological conditions of the urinary system •Explain the meaning of surgical terms related to the urinary system •Recognize common pharmacological agents used in treating the urinary system M.A.Kubtan 3 Structure and Function The Urinary System Bladder Kidneys •Also called the excretory system •Maintains water Urinary System balance •Removes waste products from the Urethra Ureters blood by excreting them in the urine Meatus M.A.Kubtan 4 Kidneys Kidneys The kidneys are bean-shaped organs located in the retroperitoneal portion of the abdominal cavity on either side of the vertebral column. Two Primary Functions •To form urine for excretion •To retain essential substances the body needs in the process called reabsorption M.A.Kubtan 5 Parts of the Kidney Kidneys filter about 1700 kidney liters of blood daily in the average adult. medulla Parts of the kidneys •Cortex hilum -outer protective portion •Medulla -inner soft portion •Hilum -a depression located in the middle of the concave side of the kidney where blood vessels, nerves, and the ureters enter and cortex exit the kidneys M.A.Kubtan 6 Urine Production Urine is produced by filtration of: •water •sugar •creatine •salts •urea •uric acid Each kidney contains more than 1 million nephrons which are the functional units of the kidneys. -
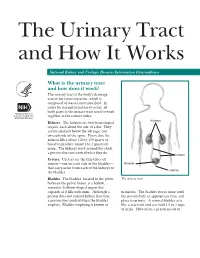
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -
GFR (Glomerular Filtration Rate) a Key to Understanding How Well Your Kidneys Are Working
GFR (Glomerular Filtration Rate) A Key to Understanding How Well Your Kidneys Are Working www.kidney.org About the Information in this Booklet Did you know that the National Kidney Foundation (NKF) offers guidelines and commentaries that help your healthcare provider make decisions about your medical treatment? The information in this booklet is based on those recommended guidelines. Stages of Kidney Disease There are five stages of kidney disease. They are shown in the table below. Your healthcare provider determines your stage of kidney disease, based on the presence of kidney damage and your glomerular filtration rate (GFR), which is a measure of your kidney function. Your treatment is based on your stage of kidney disease. Speak to your healthcare provider if you have any questions about your stage of kidney disease or your treatment. STAGES OF KIDNEY DISEASE Glomerular Stage Description Filtration Rate (GFR)* Kidney damage (e.g., protein 1 90 or above in the urine) with normal GFR Kidney damage with mild 2 60 to 89 decrease in GFR 3 Moderate decrease in GFR 30 to 59 4 Severe reduction in GFR 15 to 29 5 Kidney failure Less than 15 *Your GFR number tells your healthcare provider how much kidney function you have. As chronic kidney disease progresses, your GFR number decreases. 2 NATIONAL KIDNEY FOUNDATION Why is GFR Important? Most people are aware that their blood pressure and cholesterol numbers are important in knowing their risk for heart and blood vessel disease. Yet few know about glomerular filtration rate (GFR), one of the numbers that tells them about the health of their kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Anatomy and Physiology Male Reproductive System References
DEWI PUSPITA ANATOMY AND PHYSIOLOGY MALE REPRODUCTIVE SYSTEM REFERENCES . Tortora and Derrickson, 2006, Principles of Anatomy and Physiology, 11th edition, John Wiley and Sons Inc. Medical Embryology Langeman, pdf. Moore and Persaud, The Developing Human (clinically oriented Embryologi), 8th edition, Saunders, Elsevier, . Van de Graff, Human anatomy, 6th ed, Mcgraw Hill, 2001,pdf . Van de Graff& Rhees,Shaum_s outline of human anatomy and physiology, Mcgraw Hill, 2001, pdf. WHAT IS REPRODUCTION SYSTEM? . Unlike other body systems, the reproductive system is not essential for the survival of the individual; it is, however, required for the survival of the species. The RS does not become functional until it is “turned on” at puberty by the actions of sex hormones sets the reproductive system apart. The male and female reproductive systems complement each other in their common purpose of producing offspring. THE TOPIC : . 1. Gamet Formation . 2. Primary and Secondary sex organ . 3. Male Reproductive system . 4. Female Reproductive system . 5. Female Hormonal Cycle GAMET FORMATION . Gamet or sex cells are the functional reproductive cells . Contain of haploid (23 chromosomes-single) . Fertilizationdiploid (23 paired chromosomes) . One out of the 23 pairs chromosomes is the determine sex sex chromosome X or Y . XXfemale, XYmale Gametogenesis Oocytes Gameto Spermatozoa genesis XY XX XX/XY MALE OR FEMALE....? Male Reproductive system . Introduction to the Male Reproductive System . Scrotum . Testes . Spermatic Ducts, Accessory Reproductive Glands,and the Urethra . Penis . Mechanisms of Erection, Emission, and Ejaculation The urogenital system . Functionally the urogenital system can be divided into two entirely different components: the urinary system and the genital system. -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm.