Chronic Cassava Toxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cyanogenic Polymorphism in Trifolium Repens L
Heredity66 (1991) 105—115 Received 16 May 1990 Genetical Society of Great Britain The cyanogenic polymorphism in Trifolium repens L. (white clover) M. A. HUGHES Department of Biochemistry and Genetics, The Medical School, The University, Newcastle upon Tyne NE2 4HH Thecyanogenic polymorphism in white clover is controlled by alleles of two independently segregating loci. Biochemical studies have shown that non-functional alleles of the Ac locus, which controls the level of cyanoglucoside produced in leaf tissue, result in the loss of several steps in the biosynthetic pathway. Alleles of the Li locus control the synthesis of the hydrolytic enzyme, linamarase, which is responsible for HCN release following tissue damage. Studies on the selective forces and the distribution of the cyanogenic morphs of white clover are discussed in relation to the quantitative variation in cyanogenesis revealed by biochemical studies. Molecular studies reveal considerable restriction fragment length polymorphism for linamarase homologous genes. Keywords:cyanogenesis,polymorphism, Trifolium repen, white clover. genetics to plant taxomony. This review discusses the Introduction extensive and diverse ecological genetic studies in rela- Theterm cyanogenesis describes the release of hydro- tion to the more recent biochemical and molecular cyanic acid (HCN), which occurs when the tissues of studies of cyanogenesis in white clover. some plant species are damaged. The first report of cyanogenesis in Trifolium repens (white clover) was by Mirande (1912) and this was shortly followed by a Biochemistry paper which demonstrated that the species was poly- Theproduction of HCN by higher plants depends morphic for the character, with both cyanogenic and upon the co-occurrence of a cyanogenic glycoside and acyanogenic plants occurring in the same population catabolic enzymes. -

Chronic Cassava Toxicity
4/63 7L ARCHIV NESTEL C-010e 26528 II Chronic Cassava Toxicity Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: Barry Nestel and Reginald Maclntyre INTERNATIONAL CENTRE DE RECHERCHES DEVELOPMENT POUR LE DEVELOPPEMENT RESEARCH CENTRE INTERNATIONAL Oawa, Canada 1973 IDRC-OlOe CHRONIC CASSAVA TOXICITY Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: BARRY NESTEL AND REGINALD MACINTYRE 008817 UDC: 615.9:547.49 633.68 © 1973 International Development Research Centre Head Office: Box 8500, Ottawa, Canada. K1G 3H9 Microfiche Edition S 1 Contents Foreword Barry Nestel 5-7 Workshop Participants 8-10 Current utilization and future potential for cassava Barry Nestel 11-26 Cassava as food: toxicity and technology D. G. Coursey 27-36 Cyanide toxicity in relation to the cassava research program of CIAT in Colombia James H. Cock 37-40 Cyanide toxicity and cassava research at the International Institute of Tropical Agriculture, Ibadan, Nigeria Sidki Sadik and Sang Ki Hahn 41-42 The cyanogenic character of cassava (Manihor esculenta) G. H. de Bruijn 43-48 The genetics of cyanogenesis Monica A. Hughes 49-54 Cyanogenic glycosides: their occurrence, biosynthesis, and function Eric E. Conn 55-63 Physiological and genetic aspects of cyanogenesis in cassava and other plants G. W. Butler, P. F. Reay, and B. A. Tapper 65-71 Biosynthesis of cyanogenic glucosides in cassava (Manihot spp.) Frederick Nartey 73-87 Assay methods for hydrocyanic acid in plant tissues and their application in studies of cyanogenic glycosides in Manihot esculenta A. Zitnak 89-96 The mode of cyanide detoxication 0. -
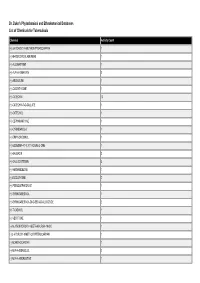
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

The Role of Phytotoxic and Antimicrobial Compounds of Euphorbia Gummifera in the Cause and Maintenance of the Fairy Circles of Namibia
The role of phytotoxic and antimicrobial compounds of Euphorbia gummifera in the cause and maintenance of the fairy circles of Namibia by Nicole Galt Submitted in partial fulfillment of the requirements for the degree Magister Scientiae Department of Plant and Soil Sciences Faculty of Natural and Agricultural Sciences University of Pretoria Pretoria Supervisor: Prof. J.J.M. Meyer March 2018 i The role of phytotoxic and antimicrobial compounds of Euphorbia gummifera in the cause and maintenance of the fairy circles of Namibia by Nicole Galt Department of Plant and Soil Sciences Faculty of Natural and Agricultural Sciences University of Pretoria Pretoria Supervisor: Prof. J.J.M. Meyer Degree: MSc Medicinal Plant Science Abstract Fairy circles (FC) are unexplained botanical phenomena of the pro-Namib desert and parts of the West Coast of South Africa. They are defined as circular to oval shaped anomalies of varying sizes that are left bereft of vegetation. Even though there are several distinctly different hypotheses that have aimed to explain the origin of fairy circles, none have done so to satisfaction of the scientific community. The aim of this study was to determine if phytotoxic and antibacterial properties of a co-occurring Euphorbia species, E. gummifera plays a role in the creation of fairy circles. Representative soil samples (from inside-, outside fairy circles and underneath dead E. gummifera plants) and plant samples (aerial ii parts of E. gummifera and intact grasses, Stipagrostis uniplumis) were collected from the area. The collected samples were used for a several biological assays. A soil bed bio-assay was done using the three collected soil types. -

The Response of Cyanogenic and Acyanogenic Phenotypes of Trifolium Repens to Soil Moisture Supply W
THE RESPONSE OF CYANOGENIC AND ACYANOGENIC PHENOTYPES OF TRIFOLIUM REPENS TO SOIL MOISTURE SUPPLY W. FOULDS Science Department, Dudley CollegeofEducation and J. P. GRIME DepartmentofBotany, University of Sheffield Received14.vi.71 I. INTRODUCTION THE legumes Trjfolium repens L. and LotuscorniculatusL. are represented in many parts of the world by populations in which some or all of the plants are capable of releasing hydrocyanic acid under certain conditions. For both species, there is evidence (Barber, 1955; Jones, 1962) that cyanogenic individuals are less susceptible to grazing by small herbivores. Cyanogenesis is determined by two independent genes designated Ac and Li (Corkill, 1942; Atwood and Sullivan, 1943). The gene Ac is dominant and is responsible for the production of two cyanogenic glucosides, lotaus- tralin and linamarin, which occur in the proportion 4 : 1 (Melville and Doak, 1940). Modifying genes determine the quantity of glucoside produced (Corkill, 1940). The cyanogenic glucosides can be hydrolysed by the - glucosidase,linamarase (Coop, 1940) the production of which is governed by the dominant gene Li. Hydrolysis yields acetone (from linamarin), methyl-ethyl-ketone (from lotaustralin), glucose, hydrogen cyanide and water. Hughes (1968) maintains that two -glucosidases occur in Trjfolium repens, one, linamarase of high activity, the other of low activity. Corkill (1940) showed that individual plants may contain both enzyme and glucoside (AcLi), enzyme only (acLi), glucoside only (Acli) or neither (acli). Daday (1954a, b, 1958) measured the contribution of each of the four phenotypes to populations of Trfo1ium repens sampled on a world scale and found that high frequencies of both Ac and Li genes were associated with warm winter conditions. -

PHYSIOLOGICAL STUDIES on the CYANOGENIC GLUCOSIDES in LOTUS ARABICUS L. Very Little Work Relating to the Physiological Studies O
PHYSIOLOGICAL STUDIES ON THE CYANOGENIC GLUCOSIDES IN LOTUS ARABICUS L. YASH PAL ABROL Division of I!lant Physiology and Phytotron, Indian A,£;ricultural Research Institute, Delhi-12 SuMMARY Observations on the occurrence of cyanogenic glucosides, linamarin and lotaustralin, in Lotus arabicus L., are reported. During the vegetative stage, a major portion of the cyanogens is localised in the leaves. With the onset of flowering, cyanogen content on per plant basis shows a sharp decrease but flower buds and flowers have a high content. Experiments on feeding carbon-14 labelled L-valine and L-isoleucine to flower buds reveals that the compounds are synthesised in these parts. lYiature seeds do not contain any detectable amounts of the cyanogens. I:-!TRODUCTION Very little work relating to the physiological studies on cyanogenic glucosides in higher plants has been reported. This is very likely due to the predominant view held until recently that a very small number of plants are cyanophoric and that cyano genic glucosides are inert end products of metabolism (Robinson, 1930). Work of physiological nature is however of interest in view of the recent observations that cyanogenic glucosidcs arc widely distributed (Dilleman, 1958; Hegnaucr, 1964), HCN is metabolised by seedlings of cyanophoric as well as non-cyano phoric plants (Blumenthal-Goldschmidt, Butler and Conn, 1963 Tschiersch, 1964; Nigam and Ressler, 1964), cyanogenic gluco sides are metabolically active rather than inert end products (Abrol, Uribe and Conn, 1965; Abrol and Conn, 1966; Abrol, Conn and Stoker, 1966) and are very likely involved in the synthesis of various neurotoxic and neurolathvritic amino acids (Ressler, 1962 ; Tschiersch, 1964). -

CYP79A118 Is Associated with the Formation of Taxiphyllin in Taxus Baccata
Plant Mol Biol (2017) 95:169–180 DOI 10.1007/s11103-017-0646-0 CYP79 P450 monooxygenases in gymnosperms: CYP79A118 is associated with the formation of taxiphyllin in Taxus baccata Katrin Luck1 · Qidong Jia2 · Meret Huber1 · Vinzenz Handrick1,3 · Gane Ka‑Shu Wong4,5,6 · David R. Nelson7 · Feng Chen2,8 · Jonathan Gershenzon1 · Tobias G. Köllner1 Received: 1 March 2017 / Accepted: 2 August 2017 / Published online: 9 August 2017 © The Author(s) 2017. This article is an open access publication Abstract plant divisions containing cyanogenic glycoside-producing Key message Conifers contain P450 enzymes from the plants has not been reported so far. We screened the tran- CYP79 family that are involved in cyanogenic glycoside scriptomes of 72 conifer species to identify putative CYP79 biosynthesis. genes in this plant division. From the seven resulting full- Abstract Cyanogenic glycosides are secondary plant com- length genes, CYP79A118 from European yew (Taxus bac- pounds that are widespread in the plant kingdom. Their bio- cata) was chosen for further characterization. Recombinant synthesis starts with the conversion of aromatic or aliphatic CYP79A118 produced in yeast was able to convert L-tyros- amino acids into their respective aldoximes, catalysed by ine, L-tryptophan, and L-phenylalanine into p-hydroxyphe- N-hydroxylating cytochrome P450 monooxygenases (CYP) nylacetaldoxime, indole-3-acetaldoxime, and phenylacetal- of the CYP79 family. While CYP79s are well known in doxime, respectively. However, the kinetic parameters of the angiosperms, their occurrence in gymnosperms and other enzyme and transient expression of CYP79A118 in Nico- tiana benthamiana indicate that L-tyrosine is the preferred Accession numbers Sequence data for genes in this article substrate in vivo. -

Poster Session Abstracts 610
Pharmaceutical Biology Pharmaceutical Biology, 2012; 50(2): 537–610 2012 © 2012 Informa Healthcare USA, Inc. ISSN 1388-0209 print/ISSN 1744-5116 online 50 DOI: 10.3109/13880209.2012.658723 2 537 Poster Session Abstracts 610 00 00 0000 00 00 0000 UMU APPLIED FOR SCREENING HERB AND PLANT EXTRACTS OR PURE PHYTOCHEMICALS FOR ANTIMUTAGENIC ACTIVITY 00 00 0000 Monique Lacroix, Stéphane Caillet, Stéphane Lessard INRS-Institut Armand-Frappier, Laval, Quebec H7V1B7, Canada 1388-0209 Antimutagenic activities of twelve herb extracts and twenty two plant extracts or pure phytochemicals assessed using a method based on the umu test system for screening natural antimutagens. All herb extracts tested showed antimuta- 1744-5116 genic properties except for Italian parsley that had mutagenic activity. Sage, mint, vervaine and oregano were the most © 2012 Informa Healthcare USA, Inc. antimutagenic. With regard to the metabolites, those from most herb extracts showed antimutagenic properties and those from garlic and thyme showed very strong antimutagenic activities, while those from camomile, rosemary and 10.3109/13880209.2012.658723 tarragon showed mutagenic activities, and those from celeriac and sage showed very strong mutagenic activities. Among pure compounds, pycnogenol metabolites showed strong antimutagenic activities. NPHB 658723 INSECTICIDAL ACTIVITY OF DERRIS MALACCENSIS FROM FRENCH POLYNESIA Heinui Philippe,1 Taivini Teai,1 Maurice Wong,2 Christian Moretti,3 Phila Raharivelomanana1 1Université de la Polynésie Française, Laboratoire BIOTEM, Faa’a, 98702, French Polynesia, 2Service du Développement Rural, Papeete, 98713, French Polynesia, 3Institut de Recherche pour le Développement, Papeete, 98713, French Polynesia Derris malaccensis (G. Bentham) D. Prain, a tropical member of the Fabaceae growing in French Polynesia, was inves- tigated to determine concentrations of metabolites (rotenoids and flavonoids) with pesticidal potential. -

Metabolism of the Cyanogenic Glucosides in Developing Flax: Metabolic Analysis, and Expression Pattern of Genes
H OH metabolites OH Article Metabolism of the Cyanogenic Glucosides in Developing Flax: Metabolic Analysis, and Expression Pattern of Genes Magdalena Zuk 1,2,* , Katarzyna Pelc 1, Jakub Szperlik 1, Agnieszka Sawula 1 and Jan Szopa 2 1 Faculty of Biotechnology, Wroclaw University, Przybyszewskiego 63/77, 51-148 Wrocław, Poland; [email protected] (K.P.); [email protected] (J.S.); [email protected] (A.S.) 2 Linum Fundation, pl. Grunwaldzki 24A, 50-363 Wrocław, Poland; [email protected] * Correspondence: [email protected] Received: 18 May 2020; Accepted: 12 July 2020; Published: 14 July 2020 Abstract: Cyanogenic glucosides (CG), the monoglycosides linamarin and lotaustralin, as well as the diglucosides linustatin and neolinustatin, have been identified in flax. The roles of CG and hydrogen cyanide (HCN), specifically the product of their breakdown, differ and are understood only to a certain extent. HCN is toxic to aerobic organisms as a respiratory inhibitor and to enzymes containing heavy metals. On the other hand, CG and HCN are important factors in the plant defense system against herbivores, insects and pathogens. In this study, fluctuations in CG levels during flax growth and development (using UPLC) and the expression of genes encoding key enzymes for their metabolism (valine N-monooxygenase, linamarase, cyanoalanine nitrilase and cyanoalanine synthase) using RT-PCR were analyzed. Linola cultivar and transgenic plants characterized by increased levels of sulfur amino acids were analyzed. This enabled the demonstration of a significant relationship between the cyanide detoxification process and general metabolism. Cyanogenic glucosides are used as nitrogen-containing precursors for the synthesis of amino acids, proteins and amines. -

Download Product Insert (PDF)
PRODUCT INFORMATION Linamarin Item No. 20532 CAS Registry No.: 554-35-8 CN Formal Name: 2-(β-D-glucopyranosyloxy)-2-methyl- propanenitrile Synonyms: α-hydroxy Isobutyronitrile β-D-glucose, O Phaseolunatin HO O MF: C10H17NO6 FW: 247.2 OH Purity: ≥98% HO Supplied as: A crystalline solid OH Storage: -20°C Stability: As supplied, 2 years from the QC date provided on the Certificate of Analysis, when stored properly Laboratory Procedures Linamarin is supplied as a crystalline solid. A stock solution may be made by dissolving the linamarin in the solvent of choice. Linamarin is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of linamarin in these solvents is approximately 10, 30, and 25 mg/ml, respectively. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of linamarin can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of linamarin in PBS, pH 7.2, is approximately 2 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description Linamarin is a glucoside of acetone cyanohydrin found in the leaves and roots of cassava, lima beans, and flax.1 It is thought to function in the transport of nitrogen from plant leaves to roots in young plants but also serves as a plant defense mechanism. -

Biomarkers of Tuber Intake Xiaomin Zhou1* , Qian Gao1, Giulia Praticò1,2, Jie Chen3 and Lars Ove Dragsted1
Zhou et al. Genes & Nutrition (2019) 14:9 https://doi.org/10.1186/s12263-019-0631-0 REVIEW Open Access Biomarkers of tuber intake Xiaomin Zhou1* , Qian Gao1, Giulia Praticò1,2, Jie Chen3 and Lars Ove Dragsted1 Abstract Tubers are important crops as well as staple foods in human nutrition. Among tubers, the potato in particular has been investigated for its health effects. However, except for its contribution to energy and effects related to resistant starch, the role of potatoes and other tubers in human health is still debated. In order to establish firm evidence for the health effects of dietary tubers and processed tuber products, it is essential to assess total intake accurately. The dietary assessment in most studies relies mainly on self-reporting and may give imprecise quantitative information on dietary intakes. Biomarkers of food intake (BFIs) are useful objective means to assess intake of specific foods or may be used as an additional measure to calibrate the measurement error in dietary reports. Here, intake biomarkers for common tubers, including potatoes and heated potato products, sweet potato, cassava, yam, and Jerusalem artichoke, are reviewed according to the biomarker of food intake reviews (BFIRev) standardized protocols for review and validation. Candidate BFIs for heated potato product include α-chaconine, α- solanine, and solanidine; less evidence is available to indicate peonidin 3-caffeoylsophoroside-5-glucoside and cyanidin 3-caffeoylsophoroside-5-glucoside as putative biomarkers having high potential specificity for purple sweet potato intake; linamarin may in addition be considered as a putative BFI for cassava. Other tubers also contain toxic glycosides or common contaminants as characteristic components but their putative use as intake biomarkers is not well documented. -

An Isolated Phytomolecule
Medical Botany 5: Active compounds in plants- cont. Alkaloids • • Nitrogenous bases which are found in plants and which are commonly found in plants and which can form salts with acids. • They are present as primary, secondary, tertiary, quaternary ammonium hydrates. • Alkaloid name is given because of similarity of alkalinity. • It is usually found in plants at 0.1-10%. O In the context of an alkaloid-bearing plant, the term usually means> 0.01% alkaloid. • Alkaloid morphine first isolated from the environment (Derosne and Seguin 1803-1804, Serturner 1805) O First synthesized cone (Ladenburg 1886) O The first used striknin (Magendie 1821) • Plants often have multiple alkaloids in different amounts in similar structures. • An alkaloide can be found in more than one plant family, as well as a single plant species. • Alkaloids are usually found in plants in their own water, in the form of their salts (salts with acids such as malic acid, tartaric acid, oxalic acid, tannic acid, citric acid). • They are found in almost all parts of plants (root, crust, leaf, seed etc.) but in different amounts. This does not mean that an alkaloid will be found in all parts of a plant. Some fruits only fruit (morphine, etc., while there are poppy seeds, not in the seed), Some of them are found in leaves and flowers (not found in the seeds of nicotine tobacco plant). • Nicotine, cones, other than those without oxygen in the constructions are usually white, crystallized dust; The above two substances are liquid. • Alkaloids are almost insoluble in water as free base (atropine, morphine); Some effects of alkaloids • Alkaloids have a wide variety of effects; Some alkaloids for some effects are as follows.