An Isolated Phytomolecule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Citicoline in the Management of Traumatic Brain Injury
pharmaceuticals Review Role of Citicoline in the Management of Traumatic Brain Injury Julio J. Secades Medical Department, Ferrer, 08029 Barcelona, Spain; [email protected] Abstract: Head injury is among the most devastating types of injury, specifically called Traumatic Brain Injury (TBI). There is a need to diminish the morbidity related with TBI and to improve the outcome of patients suffering TBI. Among the improvements in the treatment of TBI, neuroprotection is one of the upcoming improvements. Citicoline has been used in the management of brain ischemia related disorders, such as TBI. Citicoline has biochemical, pharmacological, and pharmacokinetic characteristics that make it a potentially useful neuroprotective drug for the management of TBI. A short review of these characteristics is included in this paper. Moreover, a narrative review of almost all the published or communicated studies performed with this drug in the management of patients with head injury is included. Based on the results obtained in these clinical studies, it is possible to conclude that citicoline is able to accelerate the recovery of consciousness and to improve the outcome of this kind of patient, with an excellent safety profile. Thus, citicoline could have a potential role in the management of TBI. Keywords: CDP-choline; citicoline; pharmacological neuroprotection; brain ischemia; traumatic brain injury; head injury Citation: Secades, J.J. Role of 1. Introduction Citicoline in the Management of Traumatic brain injury (TBI) is among the most devastating types of injury and can Traumatic Brain Injury. result in a different profile of neurological and cognitive deficits, and even death in the most Pharmaceuticals 2021, 14, 410. -

Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity
toxins Article Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity Massimo Picardo 1 , Oscar Núñez 2,3 and Marinella Farré 1,* 1 Department of Environmental Chemistry, IDAEA-CSIC, 08034 Barcelona, Spain; [email protected] 2 Department of Chemical Engineering and Analytical Chemistry, University of Barcelona, 08034 Barcelona, Spain; [email protected] 3 Serra Húnter Professor, Generalitat de Catalunya, 08034 Barcelona, Spain * Correspondence: [email protected] Received: 5 October 2020; Accepted: 26 November 2020; Published: 28 November 2020 Abstract: This study presents the application of a suspect screening approach to screen a wide range of natural toxins, including mycotoxins, bacterial toxins, and plant toxins, in surface waters. The method is based on a generic solid-phase extraction procedure, using three sorbent phases in two cartridges that are connected in series, hence covering a wide range of polarities, followed by liquid chromatography coupled to high-resolution mass spectrometry. The acquisition was performed in the full-scan and data-dependent modes while working under positive and negative ionisation conditions. This method was applied in order to assess the natural toxins in the Ter River water reservoirs, which are used to produce drinking water for Barcelona city (Spain). The study was carried out during a period of seven months, covering the expected prior, during, and post-peak blooming periods of the natural toxins. Fifty-three (53) compounds were tentatively identified, and nine of these were confirmed and quantified. Phytotoxins were identified as the most frequent group of natural toxins in the water, particularly the alkaloids group. -

The Cyanogenic Polymorphism in Trifolium Repens L
Heredity66 (1991) 105—115 Received 16 May 1990 Genetical Society of Great Britain The cyanogenic polymorphism in Trifolium repens L. (white clover) M. A. HUGHES Department of Biochemistry and Genetics, The Medical School, The University, Newcastle upon Tyne NE2 4HH Thecyanogenic polymorphism in white clover is controlled by alleles of two independently segregating loci. Biochemical studies have shown that non-functional alleles of the Ac locus, which controls the level of cyanoglucoside produced in leaf tissue, result in the loss of several steps in the biosynthetic pathway. Alleles of the Li locus control the synthesis of the hydrolytic enzyme, linamarase, which is responsible for HCN release following tissue damage. Studies on the selective forces and the distribution of the cyanogenic morphs of white clover are discussed in relation to the quantitative variation in cyanogenesis revealed by biochemical studies. Molecular studies reveal considerable restriction fragment length polymorphism for linamarase homologous genes. Keywords:cyanogenesis,polymorphism, Trifolium repen, white clover. genetics to plant taxomony. This review discusses the Introduction extensive and diverse ecological genetic studies in rela- Theterm cyanogenesis describes the release of hydro- tion to the more recent biochemical and molecular cyanic acid (HCN), which occurs when the tissues of studies of cyanogenesis in white clover. some plant species are damaged. The first report of cyanogenesis in Trifolium repens (white clover) was by Mirande (1912) and this was shortly followed by a Biochemistry paper which demonstrated that the species was poly- Theproduction of HCN by higher plants depends morphic for the character, with both cyanogenic and upon the co-occurrence of a cyanogenic glycoside and acyanogenic plants occurring in the same population catabolic enzymes. -

Chronic Cassava Toxicity
4/63 7L ARCHIV NESTEL C-010e 26528 II Chronic Cassava Toxicity Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: Barry Nestel and Reginald Maclntyre INTERNATIONAL CENTRE DE RECHERCHES DEVELOPMENT POUR LE DEVELOPPEMENT RESEARCH CENTRE INTERNATIONAL Oawa, Canada 1973 IDRC-OlOe CHRONIC CASSAVA TOXICITY Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: BARRY NESTEL AND REGINALD MACINTYRE 008817 UDC: 615.9:547.49 633.68 © 1973 International Development Research Centre Head Office: Box 8500, Ottawa, Canada. K1G 3H9 Microfiche Edition S 1 Contents Foreword Barry Nestel 5-7 Workshop Participants 8-10 Current utilization and future potential for cassava Barry Nestel 11-26 Cassava as food: toxicity and technology D. G. Coursey 27-36 Cyanide toxicity in relation to the cassava research program of CIAT in Colombia James H. Cock 37-40 Cyanide toxicity and cassava research at the International Institute of Tropical Agriculture, Ibadan, Nigeria Sidki Sadik and Sang Ki Hahn 41-42 The cyanogenic character of cassava (Manihor esculenta) G. H. de Bruijn 43-48 The genetics of cyanogenesis Monica A. Hughes 49-54 Cyanogenic glycosides: their occurrence, biosynthesis, and function Eric E. Conn 55-63 Physiological and genetic aspects of cyanogenesis in cassava and other plants G. W. Butler, P. F. Reay, and B. A. Tapper 65-71 Biosynthesis of cyanogenic glucosides in cassava (Manihot spp.) Frederick Nartey 73-87 Assay methods for hydrocyanic acid in plant tissues and their application in studies of cyanogenic glycosides in Manihot esculenta A. Zitnak 89-96 The mode of cyanide detoxication 0. -

Behavior of Α-Tomatine and Tomatidine Against Several Genera of Trypanosomatids from Insects and Plants and Trypanosoma Cruzi
Acta Scientiarum http://periodicos.uem.br/ojs/acta ISSN on-line: 1807-863X Doi: 10.4025/actascibiolsci.v40i1.41853 BIOTECHNOLOGY Behavior of α-tomatine and tomatidine against several genera of trypanosomatids from insects and plants and Trypanosoma cruzi Adriane Feijó Evangelista1, Erica Akemi Kavati2, Jose Vitor Jankevicius3 and Rafael Andrade Menolli4* 1Centro de Pesquisa em Oncologia Molecular, Hospital de Câncer de Barretos, Barretos, São Paulo, Brazil. 2Laboratório de Genética, Instituto Butantan, São Paulo, São Paulo, Brazil. 3Departamento de Microbiologia, Universidade Estadual de Londrina, Londrina, Paraná, Brazil. 4Centro de Ciências Médicas e Farmacêuticas, Universidade Estadual do Oeste do Paraná, Rua Universitária, 2069, 85819-110, Cascavel, Paraná, Brazil. *Author for correspondence. E-mail: [email protected] ABSTRACT. Glycoalkaloids are important secondary metabolites accumulated by plants as protection against pathogens. One of them, α-tomatine, is found in high concentrations in green tomato fruits, while in the ripe fruits, its aglycone form, tomatidine, does not present a protective effect, and it is usual to find parasites of tomatoes like Phytomonas serpens in these ripe fruits. To investigate the sensitivity of trypanosomatids to the action of α-tomatine, we used logarithmic growth phase culture of 20 trypanosomatids from insects and plants and Trypanosoma cruzi. The lethal dose 50% (LD50) was determined by mixing 107 cells of the different isolates with α-tomatine at concentrations ranging from 10-3 to 10-8 M for 30 min at room temperature. The same tests performed with the tomatidine as a control showed no detectable toxicity against the same trypanosomatid cultures. The tests involved determination of the percentage (%) survival of the protozoan cultures in a Neubauer chamber using optical microscopy. -

The Limitations of DNA Interstrand Cross-Link Repair in Escherichia Coli
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 7-12-2018 The Limitations of DNA Interstrand Cross-link Repair in Escherichia coli Jessica Michelle Cole Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Biology Commons Let us know how access to this document benefits ou.y Recommended Citation Cole, Jessica Michelle, "The Limitations of DNA Interstrand Cross-link Repair in Escherichia coli" (2018). Dissertations and Theses. Paper 4489. https://doi.org/10.15760/etd.6373 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. The Limitations of DNA Interstrand Cross-link Repair in Escherichia coli by Jessica Michelle Cole A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biology Thesis Committee: Justin Courcelle, Chair Jeffrey Singer Rahul Raghavan Portland State University 2018 i Abstract DNA interstrand cross-links are a form of genomic damage that cause a block to replication and transcription of DNA in cells and cause lethality if unrepaired. Chemical agents that induce cross-links are particularly effective at inactivating rapidly dividing cells and, because of this, have been used to treat hyperproliferative skin disorders such as psoriasis as well as a variety of cancers. However, evidence for the removal of cross- links from DNA as well as resistance to cross-link-based chemotherapy suggests the existence of a cellular repair mechanism. -

Alpha-Tomatine Content in Tomato and Tomato Products Determined By
J. Agric. Food Chem. 1995, 43, 1507-151 1 1507 a-Tomatine Content in Tomato and Tomato Products Determined by HPLC with Pulsed Amperometric Detection Mendel Friedman* and Carol E. Levin Food Safety and Health Research Unit, Western Regional Research Center, Agricultural Research Service, U.S. Department of Agriculture, 800 Buchanan Street, Albany, California 94710 Tomato plants (Lycopersicon esculentum) synthesize the glycoalkaloid a-tomatine, possibly as a defense against insects and other pests. As part of an effort to improve the safety of plant foods, the usefulness of a new HPLC pulsed amperometric detection (PAD) method for the direct analysis of a-tomatine in different parts of the tomato plant; in store-bought and field-grown, including transgenic, tomatoes; in a variety of commercial and home-processed tomato products; and in eggplant and tomatillos was evaluated. The method was found to be useful for analysis of a variety of products including high-tomatine calyxes, flowers, leaves, roots, and stems of the tomato plant (14-130 mg/100 g of fresh weight), low-tomatine red tomatoes (0.03-0.08 mg/100 g), intermediate- tomatine tomatoes (0.1-0.8 mg/100 g), and high-tomatine fresh and processed green, including pickled and fried, tomatoes (0.9-55 mg/100 g). No experimental difficulties were encountered with extraction and analysis of tomatine in complex foods such as tomato juice, ketchup, salsa, sauce, and sun-dried tomatoes. Microwaving and frying did not significantly affect tomatine levels of tomato foods. The tomatine content of fresh market and transgenic delayed-ripening varieties was not different from the range ordinarily seen in tomato. -

Use of Substituted Cinnamic Acid Polymers to Treat AIDS
Europaisches Patentamt European Patent Office © Publication number: 0 544 321 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 92120293.3 mt . ci .5 :A61K 31/78 @ Date of filing: 27.11.92 ® Priority: 27.11.91 JP 337796/91 © Inventor: Konno, Kunio 1-33-3, Kakinokizaka, Meguro-ku © Date of publication of application: Tokyo(JP) 02.06.93 Bulletin 93/22 Inventor: Sakagami, Hiroshi Chigusadai Danchi 246, 33, Chigusadai, @ Designated Contracting States: Midori-ku CH DE FR GB IT LI NL SE Yokohama-shi, Kanagawa-ken(JP) Inventor: Kawazoe, Yutaka © Applicant: Konno, Kunio 5-14-14, Shimomeguro, Meguro-ku 1-33-3, Kakinokizaka, Meguro-ku Tokyo(JP) Tokyo(JP) Inventor: Yamamoto, Naoki Applicant: Sakagami, Hiroshi Haramachi Jutaka 501, 3-11, Ebisu Minami, Chigusadai Danchi 246, 33, Chigusadai, Midori-ku Shibuya-ku Yokohama-shi, Kanagawa-ken(JP) Tokyo(JP) Applicant: Kawazoe, Yutaka 5-14-14, Shimomeguro, Meguro-ku Tokyo(JP) 0 Representative: Hansen, Bernd, Dr.rer.nat. et Applicant: Yamamoto, Naoki al Haramachi Jutaka 501, 3-11, Ebisu Minami, Hoffmann, Eitle & Partner Patentanwalte Shibuya-ku Arabellastrasse 4 Postfach 81 04 20 Tokyo(JP) W-8000 Munchen 81 (DE) © Use of substituted cinnamic acid polymers to treat AIDS. © AIDS therapeutic agents are provided which are less toxic, have a strong anti-AIDS virus activity and comprise as an effective ingredient a dehydrogenation polymer of a cinnamic acid derivative having a phenyl group substituted with at least one hydroxyl group or a pharmaceutically acceptable salt thereof. CM 00 Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 544 321 A1 BACKGROUND OF THE INVENTION Field of the Invention 5 The present invention relates to AIDS therapeutic agents containing a dehydrogenation polymer of a substituted cinnamic acid as an effective ingredient. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
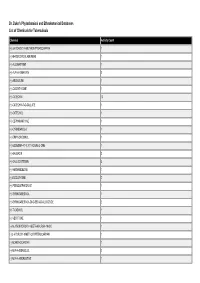
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

THE EFFECT of A-SOLANINE on ACETYLCHOLINESTERASE ACTMTY in WO: an EXAMINATION of UNDOCUMENTED BELIEFS a Thesis in Partial Fiifdi
THE EFFECT OF a-SOLANINE ON ACETYLCHOLINESTERASE ACTMTY IN WO:AN EXAMINATION OF UNDOCUMENTED BELIEFS Airdrie M. Waker A Thesis Submitted to the Faculty of Graduate Studies in partial fiifdiment of the requirements for the degree of Master of Science University of Manitoba Winnipeg, Manitoba (c) Airdrie M. Waker, 1997. National Libraiy Bibliothèque nationale du Canada Acquisitions and Acquisitions et Bibliographie Services senrices bibliographiques The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Li- of Canada to Bibliothèque nationale du Canada de reproduce, loan, distniute or sell reproduire, prêter, distn'buer ou copies of this thesis in microform, vendre des copies de cette hese sous paper or electroaic fonnats. la forme de mic~che~nim,de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. COPYRIGET PERMISSION PAGE THE EFFECT OF a-SOLANINE ON ACTEYLCROLINESTERASE ACTIVITY --IN VIVO: ANEXAKDUTIONOF UNDOCüMENTED BELIEFS A Thcrir/Pmcticum sabmittd to the Faculty of Graduate Studics of The Univenity of Manitoba in partial falfillmet of the reqaimmenti of the dm of MASTER OF SCIENCE Airdrie M. WaLker 1997 (c) Pennissiom hm ken granted to the Librnry of The University of Minitoba to Itnd or seiî copies of thu thesis/prptcticum, to the Natiooai Library of CanPàa to micronlm this thesis and to lend or sel1 copies of the mm, and to Dissertations Abstracb IaternatiooaI to pablisb an abstnct of this thesidprpcticam. -

Compendium of Botanicals Reported to Contain Naturally Occuring Substances of Possible Concern for Human Health When Used in Food and Food Supplements
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 20, 2017 brought to you by CORE provided by Online Research Database In Technology Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements EFSA authors; Pilegaard, Kirsten Link to article, DOI: 10.2903/j.efsa.2012.2663 Publication date: 2012 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): EFSA authors (2012). Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. Parma, Italy: Europen Food Safety Authority. (The EFSA Journal; No. 2663, Vol. 10(5)). DOI: 10.2903/j.efsa.2012.2663 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. EFSA Journal 2012;10(5):2663 SCIENTIFIC REPORT OF EFSA Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements1 European Food Safety Authority2, 3 European Food Safety Authority (EFSA), Parma, Italy ABSTRACT In April 2009, EFSA published on its website a Compendium of botanicals reported to contain toxic, addictive, psychotropic or other substances of concern.