Hemocyanin of the Molluscan Concholepas Concholepas Exhibits an Unusual Heterodecameric Array of Subunits*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

(Bruguiere, 1789) (Gastropoda, Muricidae) in Fjords and Channels of Southern Chile Revista Chilena De Historia Natural, Vol
Revista Chilena de Historia Natural ISSN: 0716-078X [email protected] Sociedad de Biología de Chile Chile MOLINET, CARLOS; ARÉVALO, ALEJANDRA; GONZÁLEZ, MARÍA TERESA; MORENO, CARLOS A.; ARATA, JAVIER; NIKLITSCHEK, EDWIN Patterns of larval distribution and settlement of Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) in fjords and channels of southern Chile Revista Chilena de Historia Natural, vol. 78, núm. 3, 2005, pp. 409-423 Sociedad de Biología de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=369944275005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LARVAL ECOLOGY OF C. CONCHOLEPAS IN SOUTHERNRevista CHILE Chilena de Historia Natural409 78: 409-423, 2005 Patterns of larval distribution and settlement of Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) in fjords and channels of southern Chile Patrones de distribución de larvas y asentamiento de Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) en fiordos y canales del sur de Chile CARLOS MOLINET1,3, ALEJANDRA ARÉVALO1, MARÍA TERESA GONZÁLEZ2, CARLOS A. MORENO2, JAVIER ARATA2 & EDWIN NIKLITSCHEK1 1Centro Universitario de la Trapananda e 2Instituto de Ecología y Evolución, 3Instituto de Acuicultura, Universidad Austral de Chile, Casilla 567, Valdivia, Chile; e-mail: [email protected] ABSTRACT The distribution of Concholepas concholepas (Mollusca, Gastropoda, Muricidae) is limited to the coasts of Chile and southern Peru. Almost all studies of this gastropod have been carried out in open coastal systems, rather than the fjords and channels of southern Chile, despite the fact that this area represents ca. -
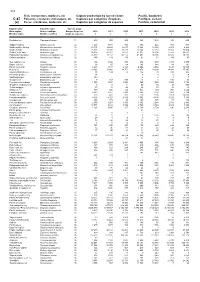
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Marine Mollusca of Isotope Stages of the Last 2 Million Years in New Zealand
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/232863216 Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia) Article in Journal- Royal Society of New Zealand · March 2011 DOI: 10.1080/03036758.2011.548763 CITATIONS READS 19 690 1 author: Alan Beu GNS Science 167 PUBLICATIONS 3,645 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Integrating fossils and genetics of living molluscs View project Barnacle Limestones of the Southern Hemisphere View project All content following this page was uploaded by Alan Beu on 18 December 2015. The user has requested enhancement of the downloaded file. This article was downloaded by: [Beu, A. G.] On: 16 March 2011 Access details: Access Details: [subscription number 935027131] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 Mortimer Street, London W1T 3JH, UK Journal of the Royal Society of New Zealand Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t918982755 Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia) AG Beua a GNS Science, Lower Hutt, New Zealand Online publication date: 16 March 2011 To cite this Article Beu, AG(2011) 'Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia)', Journal of the Royal Society of New Zealand, 41: 1, 1 — 153 To link to this Article: DOI: 10.1080/03036758.2011.548763 URL: http://dx.doi.org/10.1080/03036758.2011.548763 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. -

Genetic Structure and Diversity of Squids with Contrasting Life Histories in the Humboldt Current System Estructura Y Diversidad
Genetic diversity of squids Hidrobiológica 204, 24 (): 1-0 Genetic structure and diversity of squids with contrasting life histories in the Humboldt Current System Estructura y diversidad genética de calamares con historias de vida contrastantes en el Sistema de Corrientes de Humboldt Christian Marcelo Ibáñez and Elie Poulin Instituto de Ecología y Biodiversidad, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile. Las Palmeras # 3425, Ñuñoa. Chile e-mail: [email protected] Ibáñez C. M and Elie Poulin. 204. Genetic structure and diversity of squids with contrasting life histories in the Humboldt Current System. Hidrobiológica 24 (): -0. ABSTRACT Dosidiscus gigas and Doryteuthis gahi are the most abundant squids in the Humboldt Current System (HCS). These species have contrasting life histories. To determine the genetic structure and diversity of these species, we collected samples from different places in the HCS and amplified a fragment of the mitochondrial cytochrome C oxidase I gene. The molecular analysis of D. gigas revealed low genetic diversity, absence of population structure and evidence for a demographic expansion during the transition from the last glacial period to the current interglacial. These results sug- gest that D. gigas is composed of one large population with high levels of gene flow throughout the HCS. In the case of D. gahi, the sequences indicated the presence of two population units in the HCS, one in south-central Chile and one in Peru. The Chilean unit had greater genetic diversity, suggesting that it is an old, relatively stable population. In the Peruvian unit there was less genetic diversity and evidence of a recent demographic expansion. -

Genetic Investigation of Interspecific and Intraspecific Relationships Within the Genus Rapana
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2001 Genetic Investigation of Interspecific and Intraspecific Relationships Within the Genus Rapana Arminda L. Gensler College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Genetics Commons Recommended Citation Gensler, Arminda L., "Genetic Investigation of Interspecific and Intraspecific Relationships Within the Genus Rapana" (2001). Dissertations, Theses, and Masters Projects. Paper 1539617768. https://dx.doi.org/doi:10.25773/v5-v5kh-2t15 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. GENETIC INVESTIGATIONS OF INTERSPECIFIC AND INTRASPECIFIC RELATIONSHIPS WITHIN THE GENUS RAPANA A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Arminda L. Gensler 2001 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science Arminda L. Gensler Approved, October 2001 John E.Xjraves. Ph.D. Co-Advisor Roger Mann, Ph.D. Co-Advisor Kimberlv S. Reece, Ph.D. John M. Brubaker, Ph.D. TABLE OF CONTENTS Page -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -

Os Nomes Galegos Dos Moluscos 2020 2ª Ed
Os nomes galegos dos moluscos 2020 2ª ed. Citación recomendada / Recommended citation: A Chave (20202): Os nomes galegos dos moluscos. Xinzo de Limia (Ourense): A Chave. https://www.achave.ga /wp!content/up oads/achave_osnomesga egosdos"mo uscos"2020.pd# Fotografía: caramuxos riscados (Phorcus lineatus ). Autor: David Vilasís. $sta o%ra est& su'eita a unha licenza Creative Commons de uso a%erto( con reco)ecemento da autor*a e sen o%ra derivada nin usos comerciais. +esumo da licenza: https://creativecommons.org/ icences/%,!nc-nd/-.0/deed.g . Licenza comp eta: https://creativecommons.org/ icences/%,!nc-nd/-.0/ ega code. anguages. 1 Notas introdutorias O que cont!n este documento Neste recurso léxico fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas) ! primeira edición d" Os nomes galegos dos moluscos é do ano #$%& Na segunda edición (2$#$), adicionáronse algunhas especies, asignáronse con maior precisión algunhas das denominacións vernáculas galegas, corrixiuse algunha gralla, rema'uetouse o documento e incorporouse o logo da (have. )n total, achéganse nomes galegos para *$+ especies de moluscos A estrutura )n primeiro lugar preséntase unha clasificación taxonómica 'ue considera as clases, ordes, superfamilias e familias de moluscos !'uí apúntanse, de maneira xeral, os nomes dos moluscos 'ue hai en cada familia ! seguir -
Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Published July 20 Mar Ecol Prog Ser 1 Effects of human activities on long-term trends in sandy beach populations: the wedge clam Donax hanleyanus in Uruguay Omar Defeo*, Anita de Alava Instituto Nacional de Pesca, Casilla de Correo 1612, 11200 Montevideo, Uruguay ABSTRACT: A long-term analysis of the structure of a bivalve population consisting of the wedge clam Donax hanleyanus is described for an exposed temperate sandy beach of Uruguay. The potential effects of human harvesting on the sympatric bivalve Mesodesma mactroides (yellow clam) and of salinity were analyzed through time and space (longshore variation). Inter- and intra-annual fluctua- tions of the different population components (recruits, juveniles and adults) were detected. D. han- leyanus showed uneven periods of abundance, with the occurrence of peaks of different magnitude that appeared related to fluctuations in the fishing effort targeting on the yellow clam. D. hanlepanus showed a marked longshore variability in population structure and abundance along the 22 km of sandy beach sampled Spatial variations in salinity and also in the amount of fishing effort exerted on Ad. mactroides seem to be key factors in explaining th~svariation. This study suggests that further research on sandy beach populations should include human activities as important factors affecting long-term trends. KEY WORDS: Donax - Bivalves . Sandy beach. Long-term - Human impact. Uruguay INTRODUCTION beaches, which makes up the greatest proportion of most open shores (McLachlan 1990), has usually been Exposed marine beaches are physically stressed the focus of short-termhnstantaneous research, mainly environments (sensu McLachlan 1983, 1988), and the related to comn~unitystructure and zonation (Jaramillo invertebrate populations and communities living there 1978, Donn 1987, McLachlan 1990, Jaramillo & Mc- are usually considered to be regulated mainly by Lachlan 1993).Very little is known about the long-term physical factors. -

Introduction to Marine Ecology and Conservation
New York University Version: 2/19/21 ENVST-UA/ANST-UA 323 Marine Ecology & Conservation Spring 2021, T/R 3:30-4:45pm (4 credits) Prerequisite: ENVST-UA 100 Env. Systems Science Location: Zoom Professor: Jennifer Jacquet [email protected] Office hours: T/R 4:45-5:45 or by appointment; just drop an email Office location: Zoom Course Assistant: The Course Objectives: Welcome! This course analyzes several aspects of our oceans, with particular emphasis on human impacts and examines some of the recent major threats, including lack of governance for the high seas, deep-sea mining, and the effects of climate change on aquatic animals. We will focus ecological relationships between marine organisms and their environment, with the introduction of humans as marine predators and ecological disturbers. We will review recent peer-reviewed marine ecology studies as well as popular articles to familiarize ourselves with the latest research. The first half of the course focuses more on basic ecology while the second half focuses more on anthropogenic impacts (e.g., overexploitation, pollution, invasive species, and climate change) and proposed and tested solutions. Note: To try to capture the ‘present’ nature of the classroom, I ask that all students attend class in real time unless you have an exception (due to time zone, etc.) and, whenever possible, please have your camera on. This is also to honor the guests who will join us and who are happier when speaking to interested faces, as opposed to black boxes. Required texts: The peer-reviewed research articles listed on the daily calendar (all available on NYU Classes) and one film and one book (which you will likely need to purchase).