Introduction Laevistrombus Canarium (Linnaeus 1758)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environment for Development Improving Utilization of the Queen
Environment for Development Discussion Paper Series December 2020 ◼ EfD DP 20-39 Improving Utilization of the Queen Conch (Aliger Gigas) Resource in the Colombian Caribbean A Bioeconomic Model of Rotational Harvesting Jorge Marco, Diego Valderrama, Mario Rueda, and Maykol R o dr i g ue z - P r i et o Discussion papers are research materials circulated by their authors for purposes of information and discussion. They have not necessarily undergone formal peer review. Central America Chile China Research Program in Economics and Research Nucleus on Environmental and Environmental Economics Program in China Environment for Development in Central Natural Resource Economics (NENRE) (EEPC) America Tropical Agricultural Research and Universidad de Concepción Peking University Higher Education Center (CATIE) Colombia Ghana The Research Group on Environmental, Ethiopia The Environment and Natural Resource Natural Resource and Applied Economics Environment and Climate Research Center Research Unit, Institute of Statistical, Social Studies (REES-CEDE), Universidad de los (ECRC), Policy Studies Institute, Addis and Economic Research, University of Andes, Colombia Ababa, Ethiopia Ghana, Accra India Kenya Nigeria Centre for Research on the Economics of School of Economics Resource and Environmental Policy Climate, Food, Energy, and Environment, University of Nairobi Research Centre, University of Nigeria, (CECFEE), at Indian Statistical Institute, Nsukka New Delhi, India South Africa Tanzania Sweden Environmental Economics Policy Research Environment -

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Auckland Shell Club Auction Lot List - 22 October 2016 Albany Hall
Auckland Shell Club Auction Lot List - 22 October 2016 Albany Hall. Setup from 9am. Viewing from 10am. Auction starts at 12am Lot Type Reserve 1 WW Helmet medium size ex Philippines (John Hood Alexander) 2 WW Helmet medium size ex Philippines (John Hood Alexander) 3 WW Helmet really large ex Philippines, JHA 4 WW Tridacna (small) embedded in coral ex Tonga 1963 5 WW Lambis truncata sebae ex Tonga 1979 6 WW Charonia tritonis - whopper 45cm. No operc. Tongatapu 1979 7 WW Cowries - tray of 70 lots 8 WW All sorts but lots of Solemyidae 9 WW Bivalves 25 priced lots 10 WW Mixed - 50 lots 11 WW Cowries tray of 119 lots - some duplication but includes some scarcer inc. draconis from the Galapagos, scurra from Somalia, chinensis from the Solomons 12 WW Univalves tray of 50 13 WW Univalves tray of 57 with nice Fasciolaridae 14 WW Murex - (8) Chicoreus palmarosae, Pternotus bednallii, P. Acanthopterus, Ceratostoma falliarum, Siratus superbus, Naquetia annandalei, Murex nutalli and Hamalocantha zamboi 15 WW Bivalves - tray of 50 16 WW Bivalves - tray of 50 17 Book The New Zealand Sea Shore by Morton and Miller - fair condition 18 Book Australian Shells by Wilson and Gillett excellent condition apart from some fading on slipcase 19 Book Shells of the Western Pacific in Colour by Kira (Vol.1) and Habe (Vol 2) - good condition 20 Book 3 on Pectens, Spondylus and Bivalves - 2 ex Conchology Section 21 WW Haliotis vafescous - California 22 WW Haliotis cracherodi & laevigata - California & Aus 23 WW Amustum bellotia & pleuronecles - Queensland 24 WW Haliotis -

Nautiloid Shell Morphology
MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOWER STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOIVER 1964 STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS EXOFFICIO THEHONORABLEJACKM.CAMPBELL ................................ Governor of New Mexico LEONARDDELAY() ................................................... Superintendent of Public Instruction APPOINTEDMEMBERS WILLIAM G. ABBOTT ................................ ................................ ............................... Hobbs EUGENE L. COULSON, M.D ................................................................. Socorro THOMASM.CRAMER ................................ ................................ ................... Carlsbad EVA M. LARRAZOLO (Mrs. Paul F.) ................................................. Albuquerque RICHARDM.ZIMMERLY ................................ ................................ ....... Socorro Published February 1 o, 1964 For Sale by the New Mexico Bureau of Mines & Mineral Resources Campus Station, Socorro, N. Mex.—Price $2.50 Contents Page ABSTRACT ....................................................................................................................................................... 1 INTRODUCTION -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

Population and Reproductive Biology of the Channeled Whelk, Busycotypus Canaliculatus, in the US Mid-Atlantic
W&M ScholarWorks VIMS Articles 2017 Population and Reproductive Biology of the Channeled Whelk, Busycotypus canaliculatus, in the US Mid-Atlantic Robert A. Fisher Virginia Institute of Marine Science, [email protected] David Rudders Virginia Institute of Marine Science, [email protected] Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Marine Biology Commons Recommended Citation Fisher, Robert A. and Rudders, David, "Population and Reproductive Biology of the Channeled Whelk, Busycotypus canaliculatus, in the US Mid-Atlantic" (2017). VIMS Articles. 304. https://scholarworks.wm.edu/vimsarticles/304 This Article is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Journal of Shellfish Research, Vol. 36, No. 2, 427–444, 2017. POPULATION AND REPRODUCTIVE BIOLOGY OF THE CHANNELED WHELK, BUSYCOTYPUS CANALICULATUS, IN THE US MID-ATLANTIC ROBERT A. FISHER* AND DAVID B. RUDDERS Virginia Institute of Marine Science, College of William and Mary, PO Box 1346, Gloucester Point, VA 23062 ABSTRACT Channeled whelks, Busycotypus canaliculatus, support commercial fisheries throughout their range along the US Atlantic seaboard. Given the modest amounts of published information available on channeled whelk, this study focuses on understanding the temporal and spatial variations in growth and reproductive biology in the Mid-Atlantic region. Channeled whelks were sampled from three inshore commercially harvested resource areas in the US Mid-Atlantic: Ocean City, MD (OC); Eastern Shore of Virginia (ES); and Virginia Beach, VA (VB). The largest whelk measured 230-mm shell length (SL) and was recorded from OC. -

Impact of the the COVID-19 Pandemic on a Queen Conch (Aliger Gigas) Fishery in the Bahamas
Impact of the the COVID-19 pandemic on a queen conch (Aliger gigas) fishery in The Bahamas Nicholas D. Higgs Cape Eleuthera Institute, Rock Sound, Eleuthera, Bahamas ABSTRACT The onset of the coronavirus (COVID-19) pandemic in early 2020 led to a dramatic rise in unemployment and fears about food-security throughout the Caribbean region. Subsistence fisheries were one of the few activities permitted during emergency lockdown in The Bahamas, leading many to turn to the sea for food. Detailed monitoring of a small-scale subsistence fishery for queen conch was undertaken during the implementation of coronavirus emergency control measures over a period of twelve weeks. Weekly landings data showed a surge in fishing during the first three weeks where landings were 3.4 times higher than subsequent weeks. Overall 90% of the catch was below the minimum legal-size threshold and individual yield declined by 22% during the lockdown period. This study highlights the role of small-scale fisheries as a `natural insurance' against socio-economic shocks and a source of resilience for small island communities at times of crisis. It also underscores the risks to food security and long- term sustainability of fishery stocks posed by overexploitation of natural resources. Subjects Aquaculture, Fisheries and Fish Science, Conservation Biology, Marine Biology, Coupled Natural and Human Systems, Natural Resource Management Keywords Fisheries, Coronavirus, COVID-19, IUU, SIDS, SDG14, Food security, Caribbean, Resiliance, Small-scale fisheries Submitted 2 December 2020 INTRODUCTION Accepted 16 July 2021 Published 3 August 2021 Subsistence fishing has played an integral role in sustaining island communities for Corresponding author thousands of years, especially small islands with limited terrestrial resources (Keegan et Nicholas D. -

Extreme Ecological Specialization in a Rainforest Mammal, the Bornean
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 3 4 Extreme ecological specialization in a rainforest mammal, 5 the Bornean tufted ground squirrel, Rheithrosciurus macrotis 6 7 8 Andrew J. Marshall1*, Erik Meijaard2, and Mark Leighton3 9 10 1Department of Anthropology, Department of Ecology and Evolutionary Biology, Program in the 11 Environment, and School for Environment and Sustainability, 101 West Hall, 1085 S. University 12 Ave, Ann Arbor, Michigan, 48109 USA. 13 2Borneo Futures, Block C, Unit C8, Second Floor, Lot 51461, Kg Kota Batu, Mukim Kota Batu, 14 BA 2711, Brunei Darussalam. 15 3Harvard University, 11 Divinity Ave, Cambridge, MA, 02138, U.S.A. 16 17 * Corresponding author 18 E-mail: [email protected] (AJM) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 The endemic Bornean tufted ground squirrel, Rheithrosciurus macrotis, has attracted great 21 interest among biologists and the public recently. Nevertheless, we lack information on the most 22 basic aspects of its biology. -

AMERICAN MUSEUM NOVITATES Published by Number 1314 the AMERICAN MUSEUM of NATURAL HISTORY March 14, 1946 New York City
AMERICAN MUSEUM NOVITATES Published by Number 1314 THE AMERICAN MUSEUM OF NATURAL HISTORY March 14, 1946 New York City NOTES ON STROMBUS DENTATUS LINNE AND THE STROMBUS URCEUS COMPLEX BY HENRY DODGE A remark made by Tryon in his mono- latus Schumacher, 1817. This complex graph on the Strombidae (1885, p. 118) in presents such extremes of form, however, discussing Strombus dentatus Linn6 has that further study may justify its separa- prompted me to examine the taxonomic tion into subspecies or even species. history of the species in the so-called den- tatus group. Tryon there said: "The 1 difference between this species and S. On the first point we should turn im- urceus is so slight, and there is so much mediately to the Linnaean descriptions: variation in the shells, that it is very Strombus urceus LINNAEUS, 1758, p. 745, No. doubtful whether can be 440; 1767, p. 1212, No. 512. their separation "S. testae [sic] labro attenuato retuso brevi maintained." striato, ventre spiraque plicato-nodosis, apertura A reading of the meager references to bilabiata inermi." this group, a study of the synonyms in- TRANSLATION: Shells with a "thinned-out," of a reflected, short, and ridged lip, body-whorl and volved, and an examination consider- spire plicate-nodose, aperture bilabiate and lack- able series of specimens disclose an ob- ing armature. vious confusion which appeared as early as Strombus dentatu8 LINNAEUS, 1758, p. 745, Gmelin and has persisted in the minds of No. "o"; 1767, p. 1213, No. 513. I have "S. testa labro attenuato brevi dentato, ventre all but a few authors since his day. -

WIAD CONSERVATION a Handbook of Traditional Knowledge and Biodiversity
WIAD CONSERVATION A Handbook of Traditional Knowledge and Biodiversity WIAD CONSERVATION A Handbook of Traditional Knowledge and Biodiversity Table of Contents Acknowledgements ...................................................................................................................... 2 Ohu Map ...................................................................................................................................... 3 History of WIAD Conservation ...................................................................................................... 4 WIAD Legends .............................................................................................................................. 7 The Story of Julug and Tabalib ............................................................................................................... 7 Mou the Snake of A’at ........................................................................................................................... 8 The Place of Thunder ........................................................................................................................... 10 The Stone Mirror ................................................................................................................................. 11 The Weather Bird ................................................................................................................................ 12 The Story of Jelamanu Waterfall ......................................................................................................... -
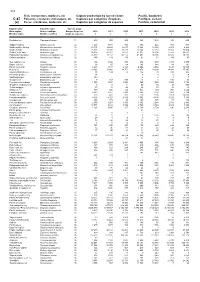
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

(Gastropoda, Strombidae) By
Mienis: Strombus terebellatus 109 Notes on the distributionand morphology of the Strombus terebellatus-complex (Gastropoda, Strombidae) by H.K. Mienis c/o Zoologisch Museum, Amsterdam data the distribu- Abbott (1960: 87-88, pi. 62) has published on tion of the Strombus (Canarium) terebellatus-complex in the Indo- Pacific. He divided this species into two geographical races: Strom- bus terebellatus terebellatus Sowerby, 1842, from the Western Paci- fic, and a new subspecies S. terebellatus afrobellatus Abbott, 1960, be distin- from the east coast of Africa. The two subspecies can the characters: guished on following S. t. terebellatus (figs. 1-3, 6) S. t.afrobellatus (figs. 4-5, 7-8) half the spire one third to spire one fourth to one third of length of the shell; the length of the shell; lines fine irregular brown spiral no spiral lines inside the inside the aperture; aperture; the last whorl forms less aperture without a posterior more or canal a posterior canal, sometimes completely covering the penultimate whorl Recently the Zoologisch Museum, Amsterdam, received three shells ofStrombus terebellatus from the Red Sea; these were live collected Williams. by Mrs. Mora The specimens were taken near Obhur, 47 km north of Jeddah, Saudi Arabia, in sand over a mucky to muddy bottom, in water from 2-5 feet depth. One specimen was supplied with the operculum. In these specimens the fine, irregular, brown lines spiral inside the aperture are present. The ratio total length/ spire length of these specimens corresponds to that given for S. t. terebellatus. The measurements in mm are: total length length spire width numberof whorls 26.8 11.5 11.4 8 29.6 11.9 11.1 8 28.8 13.8 10.2 8 So we must conclude that S.