Genetic Structure and Diversity of Squids with Contrasting Life Histories in the Humboldt Current System Estructura Y Diversidad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Working Document for the ICES WGCEPH on Cephalopod Fisheries and Life History
Working Document for the ICES WGCEPH on Cephalopod Fisheries and Life History. Lisbon, Portugal, 16-19 June 2014. Analysis of the relationships between Patagonian squid (Doryteuthis gahi) abundance and environmental parameters in the SW Atlantic using GIS tools Portela J.1 and Martinez, R.2 and Fernandes, W.2,3 1 Instituto Español de Oceanografía (IEO) Centro Oceanográfico de Vigo Cabo Estay‐Canido. 36200 Vigo, Spain [email protected] 2 Alfred Wegener Institute (AWI) Bremerhaven, Germany [email protected] 3 University of Aberdeen, Main Street, Newburgh, Aberdeenshire, AB41 6AA, UK e-mail: [email protected] ABSTRACT Doryteuthis (Loligo) gahi is the second most important cephalopod species in the SW Atlantic from a fisheries point of view. This species and hence, the fisheries profitability and sustainability, are subject to large inter-annual variability in recruitment strength, mainly due to interannual and seasonal variations of the Falkland/Malvinas Current. In this working document we analyse the environmental, geographical, temporal and physical factors affecting the distribution (abundance) of D. gahi in two areas where its abundance is highest, using geographic information systems tools. We found a slight positive correlation between CPUE and longitude, as well as between CPUE and depth in the northern area, but no correlations could be found between SST and the other explanatory variables. Regarding the southern area in summer-autumn, we found that CPUE increases with longitude as we go to the east, and with latitude. We also found positive correlations between SST and CPUE, as well as with depth. In winter spring our study revealed a slight relationship between CPUE and location, and with depth. -

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -
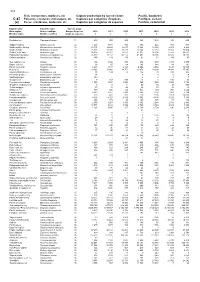
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

The Effect of Light Intensity and Tidal Cycle on the Hatching and Larval Behaviour of the Muricid Gastropod Chorus Giganteus
Journal of Experimental Marine Biology and Ecology 440 (2013) 69–73 Contents lists available at SciVerse ScienceDirect Journal of Experimental Marine Biology and Ecology journal homepage: www.elsevier.com/locate/jembe The effect of light intensity and tidal cycle on the hatching and larval behaviour of the muricid gastropod Chorus giganteus José A. Gallardo a,⁎, Antonio Brante b, Juan Miguel Cancino b a Pontificia Universidad Católica de Valparaíso, Avenida Altamirano 1480, Valparaíso, Chile b Departamento de Ecología, Facultad de Ciencias, Universidad Católica de la Santísima Concepción, Casilla 297, Concepción, Chile article info abstract Article history: Encapsulation is a common strategy observed among marine caenogastropods. Although the capsule confers Received 20 August 2012 protection for embryos, its rigidity and strength pose a significant challenge as the larvae hatch. The factors Received in revised form 19 November 2012 that drive hatching among these benthic marine gastropods have scarcely been studied. In this study, we exper- Accepted 22 November 2012 imentally evaluated whether the capsule plug opening and larval release processes were synchronised with day/ Available online xxxx night or tidal cycles in the muricid gastropod Chorus giganteus. In addition, we tested the effect of different levels of light intensity on the swimming behaviour of pre- and post-hatching larvae. The results showed that capsule Keywords: Chorus giganteus plug rupture occurred in synchronous pulses of capsule groups during both day and night. A periodogram anal- Day/night cycle ysis did not show circatidal rhythmicity in either the plug rupture or total number of capsules hatched. The Hatching highest percentage of larvae hatched at sunset and during the night (82.9%), whereas only 17% of them hatched Intracapsular development during the day. -
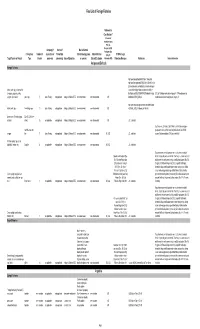
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Hemocyanin of the Molluscan Concholepas Concholepas Exhibits an Unusual Heterodecameric Array of Subunits*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 25, Issue of June 18, pp. 26134–26142, 2004 © 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Hemocyanin of the Molluscan Concholepas concholepas Exhibits an Unusual Heterodecameric Array of Subunits* Received for publication, January 28, 2004, and in revised form, April 7, 2004 Published, JBC Papers in Press, April 8, 2004, DOI 10.1074/jbc.M400903200 Pablo De Ioannes‡, Bruno Moltedo‡, Harold Oliva, Rodrigo Pacheco, Fernando Faunes, Alfredo E. De Ioannes, and Marı´a Ine´s Becker§ From the Department of Research and Development, BIOSONDA Corp., Avenida Alcalde Eduardo Castillo Velasco 2902, Santiago 7750269, Chile We describe here the structure of the hemocyanin Molluscan hemocyanins assemble into hollow cylindrical lay- from the Chilean gastropod Concholepas concholepas ered molecules formed by 10 subunits. Each subunit, ranging (CCH), emphasizing some attributes that make it inter- from 350 to 450 kDa, includes seven or eight globular folded esting among molluscan hemocyanins. CCH exhibits a domains known as functional units (FUs),1 which are arranged predominant didecameric structure as revealed by elec- into pearl-like chains and covalently bound by a short flexible tron microscopy and a size of 8 MDa by gel filtration, linker region of 10–15 amino acid residues. FUs vary in size and, in contrast with other mollusc hemocyanins, its from 45 to 55 kDa, and each of them is capable of reversibly 2؉ stabilization does not require additional Ca and/or binding one oxygen molecule through a pair of copper atoms. Downloaded from 2؉ Mg in the medium. -

Divergence of Cryptic Species of Doryteuthis Plei Blainville
Aberystwyth University Divergence of cryptic species of Doryteuthis plei Blainville, 1823 (Loliginidae, Cephalopoda) in the Western Atlantic Ocean is associated with the formation of the Caribbean Sea Sales, João Bráullio de L.; Rodrigues-Filho, Luis F. Da S.; Ferreira, Yrlene do S.; Carneiro, Jeferson; Asp, Nils E.; Shaw, Paul; Haimovici, Manuel; Markaida, Unai; Ready, Jonathan; Schneider, Horacio; Sampaio, Iracilda Published in: Molecular Phylogenetics and Evolution DOI: 10.1016/j.ympev.2016.09.014 Publication date: 2017 Citation for published version (APA): Sales, J. B. D. L., Rodrigues-Filho, L. F. D. S., Ferreira, Y. D. S., Carneiro, J., Asp, N. E., Shaw, P., Haimovici, M., Markaida, U., Ready, J., Schneider, H., & Sampaio, I. (2017). Divergence of cryptic species of Doryteuthis plei Blainville, 1823 (Loliginidae, Cephalopoda) in the Western Atlantic Ocean is associated with the formation of the Caribbean Sea. Molecular Phylogenetics and Evolution, 106(N/A), 44-54. https://doi.org/10.1016/j.ympev.2016.09.014 General rights Copyright and moral rights for the publications made accessible in the Aberystwyth Research Portal (the Institutional Repository) are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the Aberystwyth Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the Aberystwyth Research Portal Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Ink from Longfin Inshore Squid, Doryteuthis Pealeii, As a Chemical and Visual Defense Against Two Predatory Fishes, Summer Floun
CORE Metadata, citation and similar papers at core.ac.uk Provided by Woods Hole Open Access Server Reference: Biol. Bull. 225: 152–160. (December 2013) © 2013 Marine Biological Laboratory Ink From Longfin Inshore Squid, Doryteuthis pealeii, as a Chemical and Visual Defense Against Two Predatory Fishes, Summer Flounder, Paralichthys dentatus, and Sea Catfish, Ariopsis felis CHARLES D. DERBY*, MIHIKA TOTTEMPUDI, TIFFANY LOVE-CHEZEM, AND LANNA S. WOLFE Neuroscience Institute and Department of Biology, Georgia State University, Atlanta, Georgia 30303; and The Marine Biological Laboratory, Woods Hole, Massachusetts 02543 Abstract. Chemical and visual defenses are used by many Introduction organisms to avoid being approached or eaten by predators. Anti-predatory defenses can be found in many forms An example is inking molluscs—including gastropods such throughout the animal kingdom, operating through a variety as sea hares and cephalopods such as squid, cuttlefish, and of sensory systems of predators, including olfactory, visual, octopus—which release a colored ink upon approach or and auditory (Ruxton et al., 2004; Caro, 2005; Eisner et al., attack. Previous work showed that ink can protect molluscs 2007). Some molluscs use ink as a chemical defense against through a combination of chemical, visual, and other ef- predators. Previous work on slow-moving inking mol- fects. In this study, we examined the effects of ink from luscs—sea hares, Aplysia spp.—revealed a variety of mol- longfin inshore squid, Doryteuthis pealeii, on the behavior ecules acting as chemical defenses through a variety of of two species of predatory fishes, summer flounder, mechanisms (Derby, 2007; Derby and Aggio, 2011). One Paralichthys dentatus, and sea catfish, Ariopsis felis. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1.