Dissertation Modeling Landscape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
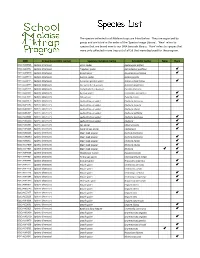
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

Leaf Herbivory by Insects During Summer Reduces Overwinter Browsing by Moose Brian P
Allman et al. BMC Ecol (2018) 18:38 https://doi.org/10.1186/s12898-018-0192-x BMC Ecology RESEARCH ARTICLE Open Access Leaf herbivory by insects during summer reduces overwinter browsing by moose Brian P. Allman, Knut Kielland and Diane Wagner* Abstract Background: Damage to plants by herbivores potentially afects the quality and quantity of the plant tissue avail- able to other herbivore taxa that utilize the same host plants at a later time. This study addresses the indirect efects of insect herbivores on mammalian browsers, a particularly poorly-understood class of interactions. Working in the Alaskan boreal forest, we investigated the indirect efects of insect damage to Salix interior leaves during the growing season on the consumption of browse by moose during winter, and on quantity and quality of browse production. Results: Treatment with insecticide reduced leaf mining damage by the willow leaf blotch miner, Micrurapteryx sali- cifoliella, and increased both the biomass and proportion of the total production of woody tissue browsed by moose. Salix interior plants with experimentally-reduced insect damage produced signifcantly more stem biomass than controls, but did not difer in stem quality as indicated by nitrogen concentration or protein precipitation capacity, an assay of the protein-binding activity of tannins. Conclusions: Insect herbivory on Salix, including the outbreak herbivore M. salicifoliella, afected the feeding behav- ior of moose. The results demonstrate that even moderate levels of leaf damage by insects can have surprisingly strong impacts on stem production and infuence the foraging behavior of distantly related taxa browsing on woody tissue months after leaves have dropped. -

Leafmining Insects Fact Sheet No
Leafmining Insects Fact Sheet No. 5.548 Insect Series|Trees and Shrubs by W.S. Cranshaw, D.A. Leatherman and J.R. Feucht* Leafminers are insects that have a habit of and/or its droppings (frass). Leaf spotting Quick Facts feeding within leaves or needles, producing fungi cause these areas to collapse, without tunneling injuries. Several kinds of insects any tunneling. • Leafminers are insects have developed this habit, including larvae of that feed within a leaf, moths (Lepidoptera), beetles (Coleoptera), producing large blotches or sawflies (Hymenoptera) and flies (Diptera). Common Leafminers meandering tunnels. Most of these insects feed for their entire of Trees and Shrubs • Although leafminer injuries larval period within the leaf. Some will also Sawfly Leafminers. Most sawflies chew are conspicuous, most pupate within the leaf mine, while others on the surface of leaves, but four species have larvae that cut their way out when full- found in Colorado develop as leafminers of leafminers produce injuries grown to pupate in the soil. woody plants. Adults are small, dark-colored, that have little, if any, effect on Leafminers are sometimes classified by non-stinging wasps that insert eggs into the plant health. the pattern of the mine which they create. newly formed leaves. The developing larvae • Most leafminers have many Serpentine leaf mines wind snake-like across produce large blotch mines in leaves during natural controls that will the leaf gradually widening as the insect late spring. The sawfly leafminers produced a normally provide good control grows. More common are various blotch single generation each year. leaf mines which are generally irregularly Elm leafminer (Kaliofenusa ulmi) is of leafminers. -

Bark Beetles Integrated Pest Management for Home Gardeners and Landscape Professionals
BARK BEETLES Integrated Pest Management for Home Gardeners and Landscape Professionals Bark beetles, family Scolytidae, are California now has 20 invasive spe- common pests of conifers (such as cies of bark beetles, of which 10 spe- pines) and some attack broadleaf trees. cies have been discovered since 2002. Over 600 species occur in the United The biology of these new invaders is States and Canada with approximately poorly understood. For more informa- 200 in California alone. The most com- tion on these new species, including mon species infesting pines in urban illustrations to help you identify them, (actual size) landscapes and at the wildland-urban see the USDA Forest Service pamphlet, interface in California are the engraver Invasive Bark Beetles, in References. beetles, the red turpentine beetle, and the western pine beetle (See Table 1 Other common wood-boring pests in Figure 1. Adult western pine beetle. for scientific names). In high elevation landscape trees and shrubs include landscapes, such as the Tahoe Basin clearwing moths, roundheaded area or the San Bernardino Mountains, borers, and flatheaded borers. Cer- the Jeffrey pine beetle and mountain tain wood borers survive the milling Identifying Bark Beetles by their Damage pine beetle are also frequent pests process and may emerge from wood and Signs. The species of tree attacked of pines. Two recently invasive spe- in structures or furniture including and the location of damage on the tree cies, the Mediterranean pine engraver some roundheaded and flatheaded help in identifying the bark beetle spe- and the redhaired pine bark beetle, borers and woodwasps. Others colo- cies present (Table 1). -

Mining Patterns of the Aspen Leaf Miner, Phyllocnistis Populiella, on Its Host Plant, Populus Tremuloides
Western North American Naturalist Volume 71 Number 1 Article 5 4-20-2011 Mining patterns of the aspen leaf miner, Phyllocnistis populiella, on its host plant, Populus tremuloides Martin L. Morton University of Tulsa, Tulsa, Oklahoma, [email protected] Maria E. Pereyra University of Tulsa, Tulsa, Oklahoma, [email protected] Follow this and additional works at: https://scholarsarchive.byu.edu/wnan Part of the Anatomy Commons, Botany Commons, Physiology Commons, and the Zoology Commons Recommended Citation Morton, Martin L. and Pereyra, Maria E. (2011) "Mining patterns of the aspen leaf miner, Phyllocnistis populiella, on its host plant, Populus tremuloides," Western North American Naturalist: Vol. 71 : No. 1 , Article 5. Available at: https://scholarsarchive.byu.edu/wnan/vol71/iss1/5 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Western North American Naturalist 71(1), © 2011, pp. 33–37 MINING PATTERNS OF THE ASPEN LEAF MINER, PHYLLOCNISTIS POPULIELLA, ON ITS HOST PLANT, POPULUS TREMULOIDES Martin L. Morton1,2 and Maria E. Pereyra1 ABSTRACT.—Feeding behavior of aspen leaf miner (Phyllocnistis populiella) larvae on quaking aspen (Populus tremu- loides) leaves was examined, by using image-analysis software, to determine the pattern and distance of the larvae’s mining trails and measure the surface area of infested leaves and consumed leaf tissue. Mining pathways between hatching and pupation sites were serpentine and generally followed predictable, sequential changes in orientation. -

Attraction of Ips Pini
CHEMICAL ECOLOGY Attraction of Ips pini (Coleoptera: Scolytinae) and Its Predators to Natural Attractants and Synthetic Semiochemicals in Northern California: Implications for Population Monitoring 1, 2 3 2 2 2 4 D. L. DAHLSTEN, D. L. SIX, D. L. ROWNEY, A. B. LAWSON, N. ERBILGIN, AND K. F. RAFFA Environ. Entomol. 33(6): 1554Ð1561 (2004) ABSTRACT Effective management of bark beetles (Coleoptera: Curculionidae, Scolytinae) relies on accurate assessments of pest and predator populations. Semiochemicals provide a powerful tool for attracting bark beetles and associated predators, but the extent to which trap catches reßect actual population densities are poorly understood. We conducted Þeld experiments in California during 2 consecutive yr to determine how attraction of Ips pini (Say) and its major predators to synthetic pheromones vary from each other and from attraction to natural volatiles emitted from colonized hosts. Synthetic lures consisted of different ratios of the (ϩ) and (Ð) enantiomers of ipsdienol, the primary pheromone component of I. pini, with or without lanierone, an additional component that synergizes attraction in some populations. I. pini was consistently attracted to either 3(ϩ)/97(Ϫ) ipsdienol or infested host plant material. Lanierone had no effect on the attraction of I. pini. Coleopteran predators showed a range of responses, more of which coincided with I. pini. Temnochila chlorodia (Mannerheim) (Trogositidae) was attracted to infested host materials and all synthetic lures. Enoclerus lecontei (Wolcott) (Cleridae) preferentially responded to higher ratios of (ϩ)-ipsdienol, and its attraction was strongly enhanced by lanierone. Enoclerus sphegeus F. was most attracted to infested hosts and exhibited a preference for (Ϫ)- over (ϩ)-ipsdienol. -

I México. Diciembre De 2015
México. Diciembre de 2015 i CODIRECTOR: ii AGRADECIMIENTOS Al Consejo Nacional de Ciencia y Tecnología (CONACYT) por financiar mi educación y brindarme la oportunidad de crecer académicamente. A la Universidad Autónoma Chapingo por brindarme enseñanzas y educación. Al. Dr. David Cibrián Tovar, por sus enseñanzas, apoyo, comprensión y aportes profesionales que contribuyeron a la construcción de este trabajo. Al Dr. Gabriel A. Rodríguez Yam por el apoyo académico, correcciones y revisiones que enriquecieron el presente trabajo. Al M. C. Rodolfo Campos Bolaños, por los comentarios, correcciones y revisiones que ayudaron al enriquecimiento de este documento. Al M. C Rolando Hernández Ruíz y a los Ing. Jesús Morales Bautista, Ing. Sergio Arturo Quiñonez Favila, Biol. Alcestis Llanderal Arango e Ing. Uriel M. Barrera Ruiz por su apoyo en la colecta y en la fase de laboratorio. Al Ph. D. Alan Burke por su ayuda como especialista en la identificación de las especies de cléridos. Al proyecto “Diagnóstico y propuesta de manejo integral para el control de insectos descortezadores en los bosques templados de la Reserva de la Biósfera Sierra Gorda (RBSG)” a cargo de la Universidad Autónoma Chapingo, en coordinación con la Agencia Alemana de Cooperación Técnica (GIZ por sus siglas en Alemán), la Comisión Nacional de Áreas Naturales Protegidas (CONANP), la Comisión Nacional Forestal (CONAFOR), Núcleos agrarios y poseedores de recursos forestales. A todas aquellas personas que contribuyeron, directa o indirectamente, en la realización de esta meta personal. iii DEDICATORIA A mis padres: Mario ( ) y Margarita A mis hermanos: Rafael, Juana, Maricela, José Alberto A mis sobrinos: Eneida, Luis, Nancy, Fabiola A mis amores del cielo: Mario ( ) y Fer ( ) A lo más hermoso de mi vida: Miguel A Abbá por pensarme iv DATOS BIOGRÁFICOS Aminta María Curiel Granados nació el 20 de abril de 1980 en el municipio de Pilcaya, Guerrero. -

Competition As a Structuring Force in Leaf Miner Communities
Oikos 118: 809Á818, 2009 doi: 10.1111/j.1600-0706.2008.17397.x, # 2009 The Authors. Journal compilation # 2009 Oikos Subject Editor: Frank Van Veen. Accepted 12 December 2008 Competition as a structuring force in leaf miner communities Ayco J. M. Tack, Otso Ovaskainen, Philip J. Harrison and Tomas Roslin A. J. M. Tack ([email protected]), O. Ovaskainen, P. J. Harrison and T. Roslin, Dept of Biological and Environmental Sciences, Div. of Population Biology, PO Box 65 (Viikinkaari 1), FI-00014 University of Helsinki, Finland. The role of competition in structuring communities of herbivorous insects is still debated. Despite this, few studies have simultaneously investigated the strength of various forms of competition and their effect on community composition. In this study, we examine the extent to which different types of competition will affect the presence and abundance of individual leaf miner species in local communities on oak trees Quercus robur. We first use a laboratory experiment to quantify the strength of intra- and interspecific competition. We then conduct a large-scale field experiment to determine whether competition occurring in one year extends to the next. Finally, we use observational field data to examine the extent to which mechanisms of competition uncovered in the two experiments actually reflect into patterns of co- occurrence in nature. In our experiment, we found direct competition at the leaf-level to be stronger among conspecific than among heterospecific individuals. Indirect competition among conspecifics lowered the survival and weight of larvae of T. ekebladella, both at the branch and the tree-level. In contrast, indirect competition among heterospecifics was only detected in one out of three species pairs examined. -

Check List of the Cleridae (Coleoptera) of Oceania
OCCASIONAL PAPERS OF BERNICE P. BISHOP MUSEUM HONOLULU, HAWAII Volume XIII 1937 Number 3 Check List of the Cleridae (Coleoptera) of Oceania By J. B. CORPORAAL ZoiiLOGISCH MUSEUM, AMSTERDAM (14th Communication on Cleridae) No more than 33 species of Cleridae, belonging to 19 genera, are known to occur in Oceania (about 2,700 species have been described). Seven of these species are not endemic, since they have a very wide distribution outside Oceania, or are even cosmopolitan, spread by commerce or by transports of plant material. Of the remaining 27 species only one belongs to an endemic (monotypic) genus, Tarso stenosis, which is closely allied to the Australian genus Tarsostenodes. Apparently no endemic species occur in Hawaii or in Samoa. All the other species belong to genera which occur in large numbers of species in the neighboring continents (or large islands as New Guinea and New Zealand). It is probable that, although the Cleridae are generally regarded as being of old descent, there are no centers of develop ment in Oceania; this is in sharp contrast to Madagascar where there are many endemic genera, with very many species, strictly confined to that island. In this list I have followed the arrangement of the Catalogue of Junk-Schenkling. I did not think it necessary to sum up all the liter ature references of the widespread species; therefore I have given only such as are necessary to recognize the species or have bearing on its variation; the biological references, however, are, as far as I could ascertain, complete up to the present. -

Widespread Mortality of Trembling Aspen (Populus Tremuloides) Throughout Interior Alaskan Boreal Forests Resulting from a Novel Canker Disease
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Plant Pathology Plant Pathology Department 2021 Widespread mortality of trembling aspen (Populus tremuloides) throughout interior Alaskan boreal forests resulting from a novel canker disease Roger W. Ruess Loretta M. Winton Gerard C. Adams Follow this and additional works at: https://digitalcommons.unl.edu/plantpathpapers Part of the Other Plant Sciences Commons, Plant Biology Commons, and the Plant Pathology Commons This Article is brought to you for free and open access by the Plant Pathology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Plant Pathology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PLOS ONE RESEARCH ARTICLE Widespread mortality of trembling aspen (Populus tremuloides) throughout interior Alaskan boreal forests resulting from a novel canker disease 1 2 3 Roger W. RuessID *, Loretta M. Winton , Gerard C. Adams 1 Institute of Arctic Biology, University of Alaska, Fairbanks, Alaska, United States of America, 2 United States Department of Agriculture Forest Service, Forest Health Protection, Fairbanks, Alaska, United States of America, 3 Department of Plant Pathology, University of Nebraska, Lincoln, Nebraska, United States of a1111111111 America a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 Abstract Over the past several decades, growth declines and mortality of trembling aspen through- out western Canada and the United States have been linked to drought, often interacting OPEN ACCESS with outbreaks of insects and fungal pathogens, resulting in a ªsudden aspen declineº Citation: Ruess RW, Winton LM, Adams GC (2021) Widespread mortality of trembling aspen throughout much of aspen's range. -

Search: Thu Nov 5 14:40:21 2020Page 1 Of
Search: Thu Nov 5 14:40:21 2020 Page 1 of 180 10 % Athalia cornubiae|[1]|GBSYM1130-12|Hymenoptera|BOLD:AAJ9512 Sciaridae|[2]|CNFNR1642-14|Diptera|BOLD:ACM8049 Sciaridae|[3]|CNPPB684-12|Diptera|BOLD:ACC8493 Sciaridae|[4]|GMGSL144-13|Diptera|BOLD:ACC8327 Sciaridae|[5]|GMOJG309-15|Diptera|BOLD:ACX6391 Tetraneura nigriabdominalis|[6]|ASHMT220-11|Hemiptera|BOLD:AAG3896 Tetraneura ulmi|[7]|CNJAE749-12|Hemiptera|BOLD:AAG3894 Prociphilus caryae|[8]|PHOCT611-11|Hemiptera|BOLD:ABY5255 Prociphilus tessellatus|[9]|TTSOW205-10|Hemiptera|BOLD:AAD7311 Grylloprociphilus imbricator|[10]|RDBA378-06|Hemiptera|BOLD:AAY2624 Subsaltusaphis virginica|[11]|RFBAC272-07|Hemiptera|BOLD:AAH9929 Euceraphis papyrifericola|[12]|BBHCN315-10|Hemiptera|BOLD:AAH2870 Euceraphis|[13]|CNPAI428-13|Hemiptera|BOLD:AAX7972 Calaphis betulaecolens|[14]|ASAHE150-12|Hemiptera|BOLD:AAC3672 Callipterinella calliptera|[15]|RDBA165-05|Hemiptera|BOLD:AAB6094 Periphyllus negundinis|[16]|CNEIB825-12|Hemiptera|BOLD:AAD3938 Sipha elegans|[17]|CNEIF3636-12|Hemiptera|BOLD:AAG1528 Aphididae|[18]|GMGSW027-13|Hemiptera|BOLD:ACD2286 Drepanaphis|[19]|RRMFE3184-15|Hemiptera|BOLD:ABY0945 Drepanaphis|[20]|RDBA009-05|Hemiptera|BOLD:AAH2879 Drepanaphis|[21]|BBHEM601-10|Hemiptera|BOLD:AAX8898 Drepanaphis|[22]|GMGSA077-12|Hemiptera|BOLD:ACA3956 Drepanaphis|[23]|ASAHE012-12|Hemiptera|BOLD:ABY1338 Drepanaphis|[24]|RRSSA5353-15|Hemiptera|BOLD:AAI6141 Drepanaphis parva|[25]|CNSLF196-12|Hemiptera|BOLD:AAX8895 Drepanaphis|[26]|CNPEE1905-14|Hemiptera|BOLD:ACL5395 Drepanaphis acerifoliae|[27]|RFBAG183-11|Hemiptera|BOLD:AAH2868 -

The Lepidoptera Families and Associated Orders of British Columbia
The Lepidoptera Families and Associated Orders of British Columbia The Lepidoptera Families and Associated Orders of British Columbia G.G.E. Scudder and R.A. Cannings March 31, 2007 G.G.E. Scudder and R.A. Cannings Printed 04/25/07 The Lepidoptera Families and Associated Orders of British Columbia 1 Table of Contents Introduction ................................................................................................................................5 Order MEGALOPTERA (Dobsonflies and Alderflies) (Figs. 1 & 2)...........................................6 Description of Families of MEGALOPTERA .............................................................................6 Family Corydalidae (Dobsonflies or Fishflies) (Fig. 1)................................................................6 Family Sialidae (Alderflies) (Fig. 2)............................................................................................7 Order RAPHIDIOPTERA (Snakeflies) (Figs. 3 & 4) ..................................................................9 Description of Families of RAPHIDIOPTERA ...........................................................................9 Family Inocelliidae (Inocelliid snakeflies) (Fig. 3) ......................................................................9 Family Raphidiidae (Raphidiid snakeflies) (Fig. 4) ...................................................................10 Order NEUROPTERA (Lacewings and Ant-lions) (Figs. 5-16).................................................11 Description of Families of NEUROPTERA ..............................................................................12