Competition As a Structuring Force in Leaf Miner Communities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final Report
Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final report Dr. Iryna Matsiakh Tbilisi 2014 This publication has been produced with the assistance of the European Union. The content, findings, interpretations, and conclusions of this publication are the sole responsibility of the FLEG II (ENPI East) Programme Team (www.enpi-fleg.org) and can in no way be taken to reflect the views of the European Union. The views expressed do not necessarily reflect those of the Implementing Organizations. CONTENTS LIST OF TABLES AND FIGURES ............................................................................................................................. 3 ABBREVIATIONS AND ACRONYMS ...................................................................................................................... 6 EXECUTIVE SUMMARY .............................................................................................................................................. 7 Background information ...................................................................................................................................... 7 Literature review ...................................................................................................................................................... 7 Methodology ................................................................................................................................................................. 8 Results and Discussion .......................................................................................................................................... -

Tischeria Decidua (Lepidoptera: Tischeriidae), New to the Belgian Fauna
Tischeria decidua (Lepidoptera: Tischeriidae), new to the Belgian fauna Willy De Prins Abstract. On 05 October 2006, two leaf mines of Tischeria decidua Wocke, 1876 were collected on Quercus robur near the ring road around Antwerpen close to Berchem railway station. Unfortunately, they only produced an Eulophid parasite. Anyway, the record stands as the leaf mineof T. decidua can easily be distinguished from the other oak mining Tischeria species. This is the first record of T. decidua known to the Belgian fauna. Samenvatting. Tischeria decidua (Lepidoptera: Tischeriidae), nieuw voor de Belgische fauna Op 05 oktober 2006 werden twee bladmijnen van Tischeria decidua Wocke, 1876 verzameld naast de Ring rond Antwerpen in de buurt van het spoorwegstation te Berchem. Jammer genoeg leverden de mijnen geen vlindertjes, maar twee parasieten uit de familie Eulophidae. De soort kan wel als nieuw voor de Belgische fauna vermeld worden omdat de bladmijn van Tischeria decidua gemakkelijk kan onderscheiden worden van de mijnen veroorzaakt door de andere Tischeria-soorten die op eik mineren. Résumé. Tischeria decidua (Lepidoptera: Tischeriidae), espèce nouvelle pour la faune belge Le 05 octobre 2006, deux feuilles minées par Tischeria decidua Wocke, 1876 furent trouvées près du ring d'Anvers, près de la gare de Berchem. Malheureusement, seulement deux parasites appartenant à la famille des Eulophidae sont éclos. L'identification de Tischeria decidua est cependant certaine puisque les mines causées par cette espèce sont très différentes de celles des autres espèces de Tischeria sur chêne. L'espèce est donc mentionnée ici pour la première fois de Belgique. Key words: Tischeria decidua – Belgium – Faunistics – First record. -

Pedunculate Oak Leaf Miners' Community
Article Pedunculate Oak Leaf Miners’ Community: Urban vs. Rural Habitat Jovan Dobrosavljevi´c 1,* , Cedomirˇ Markovi´c 1, Marija Marjanovi´c 2 and Slobodan Milanovi´c 1,3 1 Department of Forest Protection, Faculty of Forestry, University of Belgrade, Kneza Višeslava 1, 11030 Belgrade, Serbia; [email protected] (C.M.);ˇ [email protected] (S.M.) 2 Department of Landscape Horticulture, Faculty of Forestry, University of Belgrade, Kneza Višeslava 1, 11030 Belgrade, Serbia; [email protected] 3 Department of Forest Protection and Wildlife Management, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic * Correspondence: [email protected]; Tel.: +381-603-375707 Received: 6 November 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: With the process of urbanization, cities are expanding, while forests are declining. Many conditions in the urban habitats are modified compared to those in the rural ones, so the organisms present reactions to these changes. To determine to what extent the habitat type influences insects, we tested the differences in the pedunculate oak (Quercus robur L.) leaf-mining insect community between urban and rural habitats in Serbia. Lower species richness, abundance, and diversity were determined on trees in the urban environment. Due to the differences in the habitat types, many of the species disappeared, while most of the remaining species declined. The seasonal dynamics of species richness, abundance, and diversity differed between the habitat types. Both rural and urban populations started with low values in May. Subsequently, rural populations gained higher species richness, abundance, and diversity. -

INSECT SPECIES COMPOSITION on EUROPEAN CHESTNUT (Castanea Sativa Mill.) DURING FLOWERING in SELECTED LOCALITIES in SLOVAKIA
South Western Journal of Vol.10, No.2, 2019 Horticulture, Biology and Environment pp.95-104 P-Issn: 2067- 9874, E-Issn: 2068-7958 Art.no. e19107 INSECT SPECIES COMPOSITION ON EUROPEAN CHESTNUT (Castanea sativa Mill.) DURING FLOWERING IN SELECTED LOCALITIES IN SLOVAKIA Michal PÁSTOR1*, Ján KOLLÁR2 and Ladislav BAKAY2 1. National Forest Centre, Forest Research Institute Zvolen, T. G. Masaryka 22, 960 92 Zvolen, Slovakia. 2. Slovak University of Agriculture in Nitra, Faculty of Horticulture and Landscape Engineering, Department of Planting Design and Maintenance, Tulipánova 7, 949 76 Nitra, Slovakia. *Corresponding author, M. Pástor, E-mail: [email protected] ABSTRACT. The European chestnut (Castanea sativa Mill.) belongs to first introduced tree species in Slovakia. Despite being not permanent element of the Slovak flora, a different scale of insect species is associated with this plant. The paper presents, for the first time, an investigation of the insect species linked with chestnut trees in Slovakia. The monitoring of insect species composition on chestnut during the phenological growth stage of flowering was carried out in June and July 2014. Trapping and monitoring of insects was accomplished during the blooming period of flowers (catkins) on selected chestnut individuals. The research was conducted on 5 Slovakian localities: Arboretum Mlyňany, Nitra, Modrý Kameň, Dolné Plachtince and Príbelce. We recorded 70 insect species in the selected localities. They belonged to five orders (Coleoptera, Hymenoptera, Diptera, Heteroptera and Lepidoptera) and 33 families. Beetles (order Coleoptera) were the most diverse insect group with 31 species. In terms of number of individuals, orders Hymenoptera (especially Apis mellifera Linnaeus 1758 and genus Bombus sp.) and Diptera were most abundant. -
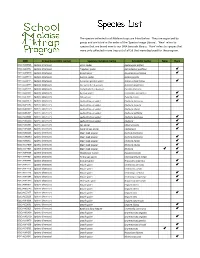
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

Leaf Herbivory by Insects During Summer Reduces Overwinter Browsing by Moose Brian P
Allman et al. BMC Ecol (2018) 18:38 https://doi.org/10.1186/s12898-018-0192-x BMC Ecology RESEARCH ARTICLE Open Access Leaf herbivory by insects during summer reduces overwinter browsing by moose Brian P. Allman, Knut Kielland and Diane Wagner* Abstract Background: Damage to plants by herbivores potentially afects the quality and quantity of the plant tissue avail- able to other herbivore taxa that utilize the same host plants at a later time. This study addresses the indirect efects of insect herbivores on mammalian browsers, a particularly poorly-understood class of interactions. Working in the Alaskan boreal forest, we investigated the indirect efects of insect damage to Salix interior leaves during the growing season on the consumption of browse by moose during winter, and on quantity and quality of browse production. Results: Treatment with insecticide reduced leaf mining damage by the willow leaf blotch miner, Micrurapteryx sali- cifoliella, and increased both the biomass and proportion of the total production of woody tissue browsed by moose. Salix interior plants with experimentally-reduced insect damage produced signifcantly more stem biomass than controls, but did not difer in stem quality as indicated by nitrogen concentration or protein precipitation capacity, an assay of the protein-binding activity of tannins. Conclusions: Insect herbivory on Salix, including the outbreak herbivore M. salicifoliella, afected the feeding behav- ior of moose. The results demonstrate that even moderate levels of leaf damage by insects can have surprisingly strong impacts on stem production and infuence the foraging behavior of distantly related taxa browsing on woody tissue months after leaves have dropped. -

Tve432 Nieukerken Johansson.Qxp
1 2 ERIK J. VAN NIEUKERKEN & ROLAND JOHANSSON 1National Museum of Natural History, Naturalis, Leiden, Netherlands 2Växjö, Sweden THE QUERCUS FEEDING STIGMELLA SPECIES OF THE WEST PALAEARCTIC: NEW SPECIES, KEY AND DISTRIBUTION (LEPIDOPTERA: NEPTICULIDAE) Nieukerken, E. J. van & R. Johansson, 2003. The Quercus feeding Stigmella species of the West Palaearctic: new species, key and distribution (Lepidoptera: Nepticulidae). – Tijdschrift voor Entomologie 146: 307-370, tables 1-3, figs. 1-179. [ISSN 0040-7496]. Published 1 December 2003. The species of the Stigmella ruficapitella group occurring in the Western Palaearctic and feed- ing on Quercus are reviewed. We recognise 19 species, five of which are described as new: Stig- mella fasciata sp. n. on Quercus pubescens from Slovenia, Croatia, Greece and Turkey, S. coc- ciferae sp. n. on Q. coccifera from Greece, Turkey and Israel, S. kasyi sp.n. from Afghanistan, possibly feeding on Quercus baloot, S. bicuspidata sp. n. from Turkey (host unknown) and S. karsholti sp. n. on Q. canariensis from Tunisia. Stigmella ilicifoliella (Mendes, 1918) comb. n., stat. rev. is removed from synonymy with S. suberivora (Stainton, 1869), it occurs in France, Spain and Portugal, partly sympatric with S. suberivora on Quercus rotundifolia, Q. ilex and Q. suber. Stigmella nigra Dufrane, 1955 is synonymised with S. ilicifoliella. S. suberivora, S. ilici- foliella, S. szoecsiella (Borkowski, 1972), S. macrolepidella (Klimesch, 1978), S. zangherii (Klimesch, 1951), S. dorsiguttella (Johansson, 1971), S. trojana Z. & A. Latuvka, 1998 and S. eberhardi (Johansson, 1971) are redescribed. A new diagnostic character on the forewing un- derside is described for male S. svenssoni (Johansson, 1971). Data on distribution and biology are given for all species, keys are given for males and for male and female genitalia. -

Leafmining Insects Fact Sheet No
Leafmining Insects Fact Sheet No. 5.548 Insect Series|Trees and Shrubs by W.S. Cranshaw, D.A. Leatherman and J.R. Feucht* Leafminers are insects that have a habit of and/or its droppings (frass). Leaf spotting Quick Facts feeding within leaves or needles, producing fungi cause these areas to collapse, without tunneling injuries. Several kinds of insects any tunneling. • Leafminers are insects have developed this habit, including larvae of that feed within a leaf, moths (Lepidoptera), beetles (Coleoptera), producing large blotches or sawflies (Hymenoptera) and flies (Diptera). Common Leafminers meandering tunnels. Most of these insects feed for their entire of Trees and Shrubs • Although leafminer injuries larval period within the leaf. Some will also Sawfly Leafminers. Most sawflies chew are conspicuous, most pupate within the leaf mine, while others on the surface of leaves, but four species have larvae that cut their way out when full- found in Colorado develop as leafminers of leafminers produce injuries grown to pupate in the soil. woody plants. Adults are small, dark-colored, that have little, if any, effect on Leafminers are sometimes classified by non-stinging wasps that insert eggs into the plant health. the pattern of the mine which they create. newly formed leaves. The developing larvae • Most leafminers have many Serpentine leaf mines wind snake-like across produce large blotch mines in leaves during natural controls that will the leaf gradually widening as the insect late spring. The sawfly leafminers produced a normally provide good control grows. More common are various blotch single generation each year. leaf mines which are generally irregularly Elm leafminer (Kaliofenusa ulmi) is of leafminers. -

Leafminers Nunn & Warrington
More dots on the map: further records of leafmining moths in East Yorkshire Andy D. Nunn1 and Barry Warrington2 1Hull International Fisheries Institute, School of Biological, Biomedical & Environmental Sciences, University of Hull, Hull, HU6 7RX, UK. Email: [email protected] 236 Marlborough Avenue, Hessle, HU13 0PN, UK. Email: [email protected] The apparent scarcity of many leafmining moths in East Yorkshire (see Sutton & Beaumont, 1989) is at least partly due to a lack of recorder effort, and a number of previously presumed scarce or rare moths are actually relatively widespread (Chesmore, 2008; Nunn, 2015). One of the aims of a previous article (Nunn, op. cit.) was, hopefully, to encourage searches for leafminers in an attempt to redress the imbalance of records in Yorkshire. This article summarises our 'leafminering' highlights from 2015. A number of sites in VC61 were searched for leafmining moths (Table 1). Sampling effort varied considerably between sites. AN’s ‘leafminering’ opportunities were mostly restricted to casual observations while on family outings; BW’s focussed on sites that could be reached using public transport. The most time was spent in the Hessle area and a productive site in Hull. In October, the authors joined Charlie Fletcher and Ian Marshall for a day in an under- recorded 10km square, concentrating on the Settrington area. North Cliffe Wood was visited only in late spring and early summer, and other sites were visited briefly in the autumn. Table 1. Most notable records by the authors of leafmining moths -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

Analyse De L'origine Des Fortes Variations D'impact De Tischeria
article Analyse de l’origine des fortes variations d’impact de Tischeria ekebladella, micro-lépidoptère minant les feuilles de Chêne, commun en Bourgogne Jean BÉGUINOT 1,2 Résumé Comme partout ailleurs, nos Chênes bourguignons hébergent de nombreux insectes dont le stade larvaire mine l’intérieur des feuilles. L’impact de ces insectes, tel qu’il peut aisément s’apprécier par la densité des mines foliaires, est éminemment variable selon les espèces mais également d’une station à l’autre (d’un chêne à un autre) pour une même espèce mineuse. Des variations d’impact d’un ordre de grandeur et parfois bien davantage sont courantes et se rencontrent au demeurant chez la plupart des insectes phytophages. Cependant, ces variations d’impact n’ont pas ordinairement pu recevoir d’explications exhaustives, intégrant l’ensemble des contributions des facteurs susceptibles d’infl uer sur le niveau d’impact. Cette limitation ne tient pas à une insuffi sance d’approche théorique mais plutôt à la quasi-impossibilité d’évaluer en direct, sur le terrain, l’ensemble des principaux facteurs infl uents, en particulier s’agissant d’insectes de petites taille, infra-centimétrique. Le recours à une procédure permettant de restituer indirectement et rétrospectivement les valeurs estimées des facteurs gouvernant le niveau d’impact (procédure ‘MELBA’), à partir de données de terrain aisément accessibles a posteriori, permet de lever commodément cette diffi culté, au moins pour le cas particulier des insectes phytophages à stade larvaire se développant aux dépens d’une seule et même feuille hôte : insectes inducteurs de galles ou fonceurs de mines. -

Redalyc.New Records of Lepidoptera from the Iberian Peninsula for 2015
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Lastuvka, A.; Lastuvka, Z. New records of Lepidoptera from the Iberian Peninsula for 2015 (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 43, núm. 172, diciembre, 2015, pp. 633-644 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45543699008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Revta. lepid., 43 (172), diciembre 2015: 633-644 eISSN: 2340-4078 ISSN: 0300-5267 New records of Lepidoptera from the Iberian Peninsula for 2015 (Insecta: Lepidoptera) A. Lastuvka & Z. Lastuvka Abstract New records of Nepticulidae, Heliozelidae, Adelidae, Tischeriidae, Gracillariidae, Argyresthiidae, Lyonetiidae and Sesiidae for Portugal and Spain are presented. Stigmella minusculella (Herrich-Schäffer, 1855), S. tormentillella (Herrich-Schäffer, 1860), Parafomoria helianthemella (Herrich-Schäffer, 1860), Antispila metallella ([Denis & Schiffermüller], 1775), Nematopogon metaxella (Hübner, [1813]), Tischeria dodonaea Stainton, 1858, Coptotriche gaunacella (Duponchel, 1843), Caloptilia fidella (Reutti, 1853), Phyllonorycter monspessulanella (Fuchs, 1897), P. spinicolella (Zeller, 1846), Lyonetia prunifoliella