COVID-19 Vaccination Communication Executive Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccination Certificate Information Version: 21 July 2021
معلومات عن شهادة التطعيم، بالعربية 中文疫苗接种凭证信息 Informations sur le certificat de vaccination en FRANÇAIS Информация о сертификате вакцинации на РУССКОМ ЯЗЫКЕ Información sobre el Certificado de Vacunación en ESPAÑOL UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME VACCINATION CERTIFICATE INFORMATION VERSION: 21 JULY 2021 ABOUT THE UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME The UN is committed to ensuring the protection of its personnel. Tasked by the Secretary-General, the Department of Operational Support (DOS) is leading a coordinated, UN-system-wide effort to ensure the availability of vaccine to UN personnel, their dependents and implementing partners. The roll-out of the UN System-wide COVID-19 Vaccination Programme (the “Programme”) for UN personnel will provide a significant boost to the ability of personnel to stay and deliver on the Organization's mandates, to support beneficiaries in the communities they serve, and to contribute to our on-going work to recover better together from the pandemic. Information about the Programme is available at: https://www.un.org/en/coronavirus/vaccination ABOUT THE VACCINATION CERTIFICATE Upon administration of the requisite vaccine dosage, a certificate of vaccination is generated through the Programme’s registration platform (the “Platform”). The certificate generated by the Platform uses a standardized format, similar to the one used by the WHO in its “International Certificate of Vaccination or Prophylaxis” (Yellow) vaccination booklet. Note: This certificate may not dispense with travel restrictions put in place by the country(ies) of destination. As the nature of the pandemic continues to rapidly evolve, it is highly advisable to check the latest travel regulations. -

Global Dynamics of a Vaccination Model for Infectious Diseases with Asymptomatic Carriers
MATHEMATICAL BIOSCIENCES doi:10.3934/mbe.2016019 AND ENGINEERING Volume 13, Number 4, August 2016 pp. 813{840 GLOBAL DYNAMICS OF A VACCINATION MODEL FOR INFECTIOUS DISEASES WITH ASYMPTOMATIC CARRIERS Martin Luther Mann Manyombe1;2 and Joseph Mbang1;2 Department of Mathematics, Faculty of Science University of Yaounde 1, P.O. Box 812 Yaounde, Cameroon 1;2;3; Jean Lubuma and Berge Tsanou ∗ Department of Mathematics and Applied Mathematics University of Pretoria, Pretoria 0002, South Africa (Communicated by Abba Gumel) Abstract. In this paper, an epidemic model is investigated for infectious dis- eases that can be transmitted through both the infectious individuals and the asymptomatic carriers (i.e., infected individuals who are contagious but do not show any disease symptoms). We propose a dose-structured vaccination model with multiple transmission pathways. Based on the range of the explic- itly computed basic reproduction number, we prove the global stability of the disease-free when this threshold number is less or equal to the unity. Moreover, whenever it is greater than one, the existence of the unique endemic equilibrium is shown and its global stability is established for the case where the changes of displaying the disease symptoms are independent of the vulnerable classes. Further, the model is shown to exhibit a transcritical bifurcation with the unit basic reproduction number being the bifurcation parameter. The impacts of the asymptomatic carriers and the effectiveness of vaccination on the disease transmission are discussed through through the local and the global sensitivity analyses of the basic reproduction number. Finally, a case study of hepatitis B virus disease (HBV) is considered, with the numerical simulations presented to support the analytical results. -

Theory at a Glance Was Published
Theory Glance at a A Guide For Health Promotion Practice (Second Edition) U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health Foreword decade ago, the first edition of Theory at a Glance was published. The guide was a welcome resource for public health practitioners seeking a single, concise summary of health behavior theories that was neither overwhelming nor superficial. As a government publication in the public domain, it also provided cash-strapped Ahealth departments with access to a seminal integration of scholarly work that was useful to program staff, interns, and directors alike. Although they were not the primary target audience, members of the public health research community also utilized Theory at a Glance, both as a quick desk reference and as a primer for their students. The National Cancer Institute is pleased to sponsor the publication of this guide, but its relevance is by no means limited to cancer prevention and control. The principles described herein can serve as frameworks for many domains of public health intervention, complementing focused evidence reviews such as Centers for Disease Control and Prevention’s Guide to Community Preventive Services. This report also complements a number of other efforts by NCI and our federal partners to facilitate more rigorous testing and application of health behavior theories through training workshops and the development of new Web-based resources. One reason theory is so useful is that it helps us articulate assumptions and hypotheses concerning our strategies and targets of intervention. Debates among policymakers concerning public health programs are often complicated by unspoken assumptions or confusion about which data are relevant. -

CIOMS Guide to Vaccine Safety Communication
2018 CIOMS Guide to Vaccine Safety Communication Report by Topic Group 3 of the CIOMS Working Group on Vaccine Safety Council for International Organizations of CIOMS Guide to Vaccine Safety Communication CIOMS Guide to Vaccine Medical Sciences (CIOMS) Geneva, Switzerland 2018 CIOMS CIOMS Guide to Vaccine Safety Communication Report by Topic Group 3 of the CIOMS Working Group on Vaccine Safety Council for International Organizations of Medical Sciences (CIOMS) Geneva,Geneva Switzerland 2014 2018 Copyright © 2018 by the Council for International Organizations of Medical Sciences (CIOMS) ISBN: 978-92-9036091-9 All rights reserved. CIOMS publications may be obtained directly from CIOMS using its website e-shop module at https://cioms.ch/shop/. Further information can be obtained from CIOMS P.O. Box 2100, CH-1211 Geneva 2, Switzerland, tel.: +41 22 791 6497, www.cioms.ch, e-mail: [email protected]. CIOMS publications are also available through the World Health Organization, WHO Press, 20 Avenue Appia, CH-1211 Geneva 27, Switzerland. Citations: CIOMS Guide to vaccine safety communication. Report by topic group 3 of the CIOMS Working Group on Vaccine Safety. Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS), 2018. Note on style: This publication uses the World Health Organization’s WHO style guide, 2nd Edition, 2013 (WHO/ KMS/WHP/13.1) wherever possible for spelling, punctuation, terminology and formatting which combines British and American English conventions. Disclaimer: The authors alone are responsible for the views expressed in this publication and those views do not necessarily represent the decisions, policies or views of their respective institutions or companies. -

Diffusion of Innovations in Service Organizations: Systematic Review and Recommendations
Diffusion of Innovations in Service Organizations: Systematic Review and Recommendations TRISHA GREENHALGH, GLENN ROBERT, FRASER MACFARLANE∗,PAULBATE, and OLIVIA KYRIAKIDOU∗ University College London; ∗University of Surrey This article summarizes an extensive literature review addressing the question, How can we spread and sustain innovations in health service delivery and or- ganization? It considers both content (defining and measuring the diffusion of innovation in organizations) and process (reviewing the literature in a sys- tematic and reproducible way). This article discusses (1) a parsimonious and evidence-based model for considering the diffusion of innovations in health service organizations, (2) clear knowledge gaps where further research should be focused, and (3) a robust and transferable methodology for systematically re- viewing health service policy and management. Both the model and the method should be tested more widely in a range of contexts. Key Words: Diffusion of innovation, systematic review, implementation. his article summarizes the findings of a systematic literature review of the diffusion of service inno- T vations. The United Kingdom Department of Health explic- itly commissioned this work, which was carried out between October 2002 and December 2003, for its National Health Service’s exten- sive modernization agenda (UK Department of Health 2001). Our review, which supplements and extends previous overviews and meta- analyses (Damanpour 1991, 1992, 1996; Granados et al. 1997; Meyers, Sivakumar, and Nakata 1999; Rogers 1995; Tornatsky and Klein 1982; Address correspondence to: Trisha Greenhalgh, University College London, Room 403, Holborn Union Building, Highgate Hill, London N19 5LW, United Kingdom (e-mail: [email protected]). The Milbank Quarterly, Vol. -

Communicating the Risks of Urban Air Pollution to the Public. a Study of Urban Air Pollution Information Services
Rev. Int. Contam. Ambie. 31 (4) 361-375, 2015 COMMUNICATING THE RISKS OF URBAN AIR POLLUTION TO THE PUBLIC. A STUDY OF URBAN AIR POLLUTION INFORMATION SERVICES Christian OLTRA* and Roser SALA Centro de Investigación Sociotécnica, Departamento de Medio Ambiente, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT). Gran Vía de las Cortes Catalanas 604, Barcelona, España, 08007 *Corresponding author: [email protected] (Received September 2014; accepted April 2015) Key words: air quality, risk communication ABSTRACT Communicating to the public about urban air pollution is a complex task. It requires careful consideration of the goals and objectives of the communication, the target audience, the type of information and the messages to be conveyed, and the vehicles through which the message will be delivered. This complexity increases when the goal of communication is not only making information about air pollution available to the public, but also to promote socially beneficial changes in the behavior of various social groups. In order to understand in greater depth the challenges of communicating dif- ferent air pollution issues, we evaluated the public air pollution information services provided by public information services in four Spanish cities, based on interviews with experts and a documentary analysis. We identified the main features in terms of five dimensions (goals of communication, type of information, communication mechanisms, intended audience and intended effects), then we explored the limitations of these information systems, and analyze the beliefs and assumptions of the experts concerning communicating with the public. We recommend that air quality manage- ment planners assess their opportunities to foster both a broader public engagement and behavioral modifications in a way that complements and extends current structural and informational interventions. -

Communication As an Essential Component of Environmental Health Science
AdVANcEmENtAd VAN c E m EN t of tHEt HE PRACTICE DIRECT FROM CDC ENVIRONMENTAL HEALTH SERVICES BRANCH Communication as an Essential Component of Environmental Health Science Ricardo R. Beato, Jana Telfer, MS MA and community health decisions. The use of Editor's Note: This is the first of two columns this month about the research adds scientific rigor to health commu Environmental Health Training in Emergency Response (EHTER) Awareness nication planning and implementation. Health Level course. NEHA strives to provide up-to-date and relevant information communication professionals are uniquely trained and qualified to conduct communica on environmental health and to build partnerships in the profession. In tion research, develop effective and duplicable pursuit of these goals, we feature a column from the Environmental Health health promotion strategies and campaigns, Services Branch (EHSB) of the Centers for Disease Control and Prevention and evaluate communication effectiveness. (CDC) in every issue of the Journal. The U.S. Department of Health and Human Services (HHS) included health communica In this column, EHSB and guest authors from across CDC will highlight a tion science among the Healthy People 2010 variety of concerns, opportunities, challenges, and successes that we all share objectives. HHS states that during the first de in environmental public health. EHSB’s objective is to strengthen the role of cade of the 21st century, health communication state, local, and national environmental health programs and professionals has been an essential contributor to improved to anticipate, identify, and respond to adverse environmental exposures and personal and community health. Public health professionals must continue to build an acces the consequences of these exposures for human health. -

Systematic Scoping Review on Social Media Monitoring Methods and Interventions Relating to Vaccine Hesitancy
TECHNICAL REPORT Systematic scoping review on social media monitoring methods and interventions relating to vaccine hesitancy www.ecdc.europa.eu ECDC TECHNICAL REPORT Systematic scoping review on social media monitoring methods and interventions relating to vaccine hesitancy This report was commissioned by the European Centre for Disease Prevention and Control (ECDC) and coordinated by Kate Olsson with the support of Judit Takács. The scoping review was performed by researchers from the Vaccine Confidence Project, at the London School of Hygiene & Tropical Medicine (contract number ECD8894). Authors: Emilie Karafillakis, Clarissa Simas, Sam Martin, Sara Dada, Heidi Larson. Acknowledgements ECDC would like to acknowledge contributions to the project from the expert reviewers: Dan Arthus, University College London; Maged N Kamel Boulos, University of the Highlands and Islands, Sandra Alexiu, GP Association Bucharest and Franklin Apfel and Sabrina Cecconi, World Health Communication Associates. ECDC would also like to acknowledge ECDC colleagues who reviewed and contributed to the document: John Kinsman, Andrea Würz and Marybelle Stryk. Suggested citation: European Centre for Disease Prevention and Control. Systematic scoping review on social media monitoring methods and interventions relating to vaccine hesitancy. Stockholm: ECDC; 2020. Stockholm, February 2020 ISBN 978-92-9498-452-4 doi: 10.2900/260624 Catalogue number TQ-04-20-076-EN-N © European Centre for Disease Prevention and Control, 2020 Reproduction is authorised, provided the -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -
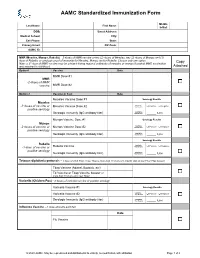
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

ADVISORY COMMISSION on CHILDHOOD VACCINES TABLE of CONTENTS March 3, 2016
ADVISORY COMMISSION ON CHILDHOOD VACCINES TABLE OF CONTENTS March 3, 2016 TAB ACCV Agenda 1 ACCV Charter ACCV Roster 2016 Meeting Dates Meeting Minutes 2 o Draft Minutes – December 3, 2015 Vaccine Injury Compensation Trust Fund Statement 3 o Vaccine Injury Trust Fund Summary Sheet for the Period of 10/1/2015 – 1/31/2016 VICP Data and Statistics 4 Meeting Presentations & Updates 5 o Impact of Increased Claims Filed 5.1 o Report from the Division of Vaccine Injury Compensation 5.2 o Report from the Department of Justice 5.3 o Vaccine Information Statements 5.4 o Polio o Varicella o Update on the Immunization Safety Office Vaccine Activities (CDC) 5.5 o Update on the National Institute of Allergy and Infectious Disease (NIH) 5.6 o Update on the Center for Biologics, Evaluation and Research (FDA) 5.7 o Update from the National Vaccine Program Office (NVPO) 5.8 o Adult Immunization Plan Program-Related Articles/Publications 6 o Forbes, “Gardasil HPV Vaccine Safety Assessed In Most Comprehensive Study 6.1 To Date” o National Geographic, “As Antibiotics Fail, We Need More Vaccines” 6.2 o CNN, “New vaccines for HPV, meningitis recommended for kids and adults” 6.3 o Forbes, “Is It Time To Ditch Tdap As A Routinely Recommended Teen 6.4 Vaccination?” ADVISORY COMMISSION ON CHILDHOOD VACCINES Agenda February 11, 2016 ADVISORY COMMISSION ON CHILDHOOD VACCINES (ACCV) Teleconference and Adobe Connect March 3, 2016 (10:00 am – 3:15 pm Eastern Daylight Time) Dial in: 1-800-779-3561 Passcode: 8164763 https://hrsa.connectsolutions.com/accv/ Time Agenda Item Presenter 10:00 AM Welcome and Chair Report Dr.