Bouvet Island), South Atlantic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MARC Code List for Countries: Part I
Code Sequence PART II: CODE SEQUENCE Discontinued codes are identified by a hyphen preceding the code aa Albania ce Sri Lanka abc Alberta cf Congo (Brazzaville) -ac Ashmore and Cartier Islands cg Congo (Democratic Republic) ae Algeria ch China (Republic : 1949- ) af Afghanistan ci Croatia ag Argentina cj Cayman Islands -ai Anguilla ck Colombia ai Armenia (Republic) cl Chile -air Armenian S.S.R. cm Cameroon aj Azerbaijan -cn Canada -ajr Azerbaijan S.S.R. cou Colorado aku Alaska -cp Canton and Enderbury Islands alu Alabama cq Comoros am Anguilla cr Costa Rica an Andorra -cs Czechoslovakia ao Angola ctu Connecticut aq Antigua and Barbuda cu Cuba aru Arkansas cv Cape Verde as American Samoa cw Cook Islands at Australia cx Central African Republic au Austria cy Cyprus aw Aruba -cz Canal Zone ay Antarctica dcu District of Columbia azu Arizona deu Delaware ba Bahrain dk Denmark bb Barbados dm Benin bcc British Columbia dq Dominica bd Burundi dr Dominican Republic be Belgium ea Eritrea bf Bahamas ec Ecuador bg Bangladesh eg Equatorial Guinea bh Belize em East Timor bi British Indian Ocean Territory enk England bl Brazil er Estonia bm Bermuda Islands -err Estonia bn Bosnia and Hercegovina es El Salvador bo Bolivia et Ethiopia bp Solomon Islands fa Faroe Islands br Burma fg French Guiana bs Botswana fi Finland bt Bhutan fj Fiji bu Bulgaria fk Falkland Islands bv Bouvet Island flu Florida bw Belarus fm Micronesia (Federated States) -bwr Byelorussian S.S.R. fp French Polynesia bx Brunei fr France cau California fs Terres australes et antarctiques cb Cambodia françaises cc China ft Djibouti cd Chad gau Georgia MARC Code List for Countries page 37 Code Sequence gb Kiribati kz Kazakhstan gd Grenada -kzr Kazakh S.S.R. -

Final Report
Time to lay down the law National legislation to enforce the BWC The Convention on the Prohibition of the Development, is the Verification Research, Training and Information Production and Stockpiling of Bacteriological (Biological) Centre, an independent, non-profit making, non-govern- Time to lay down the law and Toxin Weapons and on eir Destruction () obliges mental organisation. Its mission is to promote effective and states parties to take any necessary national measures to efficient verification as a means of ensuring confidence in National legislation to enforce the BWC implement the treaty in their territory. States parties have the implementation of international agreements and intra- agreed that this requires the adoption of legislation, including national agreements with international involvement. penal sanctions. aims to achieve its mission through research, training, Only now, thirty years after the treaty’s adoption, are states dissemination of information, and interaction with the parties collectively considering what legislative provisions relevant political, diplomatic, technical, scientific and non- are required to effectively enforce the treaty. This report is governmental communities. intended to assist states parties in this endeavour by assessing The Centre is based at Baird House, – St. Cross Street, the current status of their national legislation to enforce the London, , United Kingdom. treaty’s core prohibitions. The report provides comparative Phone +.()... analysis of existing legislation, makes recommendations for Fax +.()... increasing the rate of adoption of legislation and proposes E-mail [email protected] ways to make existing and new legislation more effective. Web www.vertic.org It should be of use to states parties preparing to adopt or amend legislation, states and organisations providing assist- Design and production: Richard Jones, Exile: Design and ance to states parties, and to the international community Editorial Services ([email protected]). -

Igneous Rocks of Peter I Island Hemisphere Tectonic Reconstructions
LeMasurier, W. E., and F. A. Wade. In press. Volcanic history in Marie Byrd Land: implications with regard to southern Igneous rocks of Peter I Island hemisphere tectonic reconstructions. In: Proceedings of the International Symposium on Andean and Antarctic Vol- canology Problems, Santiago, Chile (0. Gonzalez-Ferran, edi- tor). Rome, International Association of Volcanology and Chemistry of Earths Interior. THOMAS W. BASTIEN Price, R. C., and S. R. Taylor. 1973. The geochemistry of Dune- Ernest E. Lehmann Associates din Volcano, East Otago, New Zealand: rare earth elements. Minneapolis, Minnesota 55403 Contributions to Mineralogy and Petrology, 40: 195-205. Sun, S. S., and G. N. Hanson. 1975. Origin of Ross Island basanitoids and limitations upon the heterogeneity of mantle CAMPBELL CRADDOCK sources of alkali basalts and nephelinites. Contributions to Department of Geology and Geophysics Mineralogy and Petrology, 52: 77-106. The University of Wisconsin, Madison Sun, S. S., and G. N. Hanson. 1976. Rare earth element evi- Madison, Wisconsin 53706 dence for differentiation of McMurdo volcanics, Ross Island, Antarctica. Contributions to Mineralogy and Petrology, 54: 139-155. Peter I Island lies in the southeastern Pacific Ocean at 68°50S. 90°40W. about 240 nautical miles off the Eights Coast of West Antarctica. Ris- ing from the continental rise, it is one of the few truly oceanic islands in the region. Few people have been on the island, and little is known of its geology. Thaddeus von Bellingshausen discovered and named the island in 1821, and it was not seen again until sighted by Pierre Charcot in 1910. A Nor- wegian ship dredged some rocks off the west coast in 1927, and persons from the Norvegia achieved the first landing in 1929. -

Florida Department of Education
FLORIDA DEPARTMENT OF EDUCATION Implementation Date: DOE INFORMATION DATA BASE REQUIREMENTS Fiscal Year 1995-96 VOLUME II: AUTOMATED STAFF INFORMATION SYSTEM July 1, 1995 AUTOMATED STAFF DATA ELEMENTS APPENDIX C COUNTRY CODES CODE COUNTRY CODE COUNTRY AF Afghanistan CV Cape Verde AB Albania CJ Cayman Islands AG Algeria CP Central African Republic AN Andorra CD Chad AO Angola CI Chile AV Anguilla CH China AY Antarctica KI Christmas Island AC Antigua and Barbuda CN Clipperton Island AX Antilles KG Cocos Islands (Keeling) AE Argentina CL Colombia AD Armenia CQ Comoros AA Aruba CF Congo AS Australia CR Coral Sea Island AU Austria CS Costa Rica AJ Azerbaijan DF Croatia AI Azores Islands, Portugal CU Cuba BF Bahamas DH Curacao Island BA Bahrain CY Cyprus BS Baltic States CX Czechoslovakia BG Bangladesh DT Czech Republic BB Barbados DK Democratic Kampuchea BI Bassas Da India DA Denmark BE Belgium DJ Djibouti BZ Belize DO Dominica BN Benin DR Dominican Republic BD Bermuda EJ East Timor BH Bhutan EC Ecuador BL Bolivia EG Egypt BJ Bonaire Island ES El Salvador BP Bosnia and Herzegovina EN England BC Botswana EA Equatorial Africa BV Bouvet Island EQ Equatorial Guinea BR Brazil ER Eritrea BT British Virgin Islands EE Estonia BW British West Indies ET Ethiopia BQ Brunei Darussalam EU Europa Island BU Bulgaria FA Falkland Islands (Malvinas) BX Burkina Faso, West Africa FO Faroe Islands BM Burma FJ Fiji BY Burundi FI Finland JB Byelorussia SSR FR France CB Cambodia FM France, Metropolitian CM Cameroon FN French Guiana CC Canada FP French Polynesia Revised: -

National Report Norway
HCA9-07.4Ad IHO HYDROGRAPHIC COMMITTEE ON ANTARCTICA (HCA) 9th Meeting, Simon’s Town, Cape Town, South Africa, 12-14 October 2009 NATIONAL REPORT – NORWAY NORWEGIAN HYDROGRAPHIC SERVICE (NHS) 1. General The Norwegian Hydrographic Service (NHS) is responsible for the provision of hydrographic services for Norwegian waters and sea areas, including the Svalbard and Jan Mayen area. In Antarctica the Norwegian geographical area of interest mainly is concentrated in Dronning Maud Land (Queen Maud Land) and the adjacent Kong Håkon VII Hav (King Håkon VII Sea). Peter I Øy (Peter I Island) in the Bellingshausen Sea and Bouvetøya (Bouvet Island) are also Norwegian dependencies. However, the latter is north of the 60 parallel. In July 2008, Norway reached the goal of full ENC coverage for the Norwegian coast. Although this was a major step forwards, there still remains large areas with old data that needs to be replaced with new survey data. In addition, large areas on Svalbard remain to be surveyed and covered with ENCs and papercharts. The NHS has established a fully digital production flowline and all new products and updates are produced from a seamless Primary database. A new chart production system that enables more efficient maintenance of the ENC and chart portfolio is currently being fully implemented. The NHS intends to establish a Tracing service in 2009 and Print on Demand in 2010. Two projects are being carried out in order to develop the services including the technology needed to support it. PRIMAR International ENC service, operated by the Norwegian Hydrographic Service, now includes approx 8400 ENCs from more than 35 countries. -

Table 1 Comprehensive International Points List
Table 1 Comprehensive International Points List FCC ITU-T Country Region Dialing FIPS Comments, including other 1 Code Plan Code names commonly used Abu Dhabi 5 971 TC include with United Arab Emirates Aden 5 967 YE include with Yemen Admiralty Islands 7 675 PP include with Papua New Guinea (Bismarck Arch'p'go.) Afars and Assas 1 253 DJ Report as 'Djibouti' Afghanistan 2 93 AF Ajman 5 971 TC include with United Arab Emirates Akrotiri Sovereign Base Area 9 44 AX include with United Kingdom Al Fujayrah 5 971 TC include with United Arab Emirates Aland 9 358 FI Report as 'Finland' Albania 4 355 AL Alderney 9 44 GK Guernsey (Channel Islands) Algeria 1 213 AG Almahrah 5 967 YE include with Yemen Andaman Islands 2 91 IN include with India Andorra 9 376 AN Anegada Islands 3 1 VI include with Virgin Islands, British Angola 1 244 AO Anguilla 3 1 AV Dependent territory of United Kingdom Antarctica 10 672 AY Includes Scott & Casey U.S. bases Antigua 3 1 AC Report as 'Antigua and Barbuda' Antigua and Barbuda 3 1 AC Antipodes Islands 7 64 NZ include with New Zealand Argentina 8 54 AR Armenia 4 374 AM Aruba 3 297 AA Part of the Netherlands realm Ascension Island 1 247 SH Ashmore and Cartier Islands 7 61 AT include with Australia Atafu Atoll 7 690 TL include with New Zealand (Tokelau) Auckland Islands 7 64 NZ include with New Zealand Australia 7 61 AS Australian External Territories 7 672 AS include with Australia Austria 9 43 AU Azerbaijan 4 994 AJ Azores 9 351 PO include with Portugal Bahamas, The 3 1 BF Bahrain 5 973 BA Balearic Islands 9 34 SP include -

ISO Country Codes
COUNTRY SHORT NAME DESCRIPTION CODE AD Andorra Principality of Andorra AE United Arab Emirates United Arab Emirates AF Afghanistan The Transitional Islamic State of Afghanistan AG Antigua and Barbuda Antigua and Barbuda (includes Redonda Island) AI Anguilla Anguilla AL Albania Republic of Albania AM Armenia Republic of Armenia Netherlands Antilles (includes Bonaire, Curacao, AN Netherlands Antilles Saba, St. Eustatius, and Southern St. Martin) AO Angola Republic of Angola (includes Cabinda) AQ Antarctica Territory south of 60 degrees south latitude AR Argentina Argentine Republic America Samoa (principal island Tutuila and AS American Samoa includes Swain's Island) AT Austria Republic of Austria Australia (includes Lord Howe Island, Macquarie Islands, Ashmore Islands and Cartier Island, and Coral Sea Islands are Australian external AU Australia territories) AW Aruba Aruba AX Aland Islands Aland Islands AZ Azerbaijan Republic of Azerbaijan BA Bosnia and Herzegovina Bosnia and Herzegovina BB Barbados Barbados BD Bangladesh People's Republic of Bangladesh BE Belgium Kingdom of Belgium BF Burkina Faso Burkina Faso BG Bulgaria Republic of Bulgaria BH Bahrain Kingdom of Bahrain BI Burundi Republic of Burundi BJ Benin Republic of Benin BL Saint Barthelemy Saint Barthelemy BM Bermuda Bermuda BN Brunei Darussalam Brunei Darussalam BO Bolivia Republic of Bolivia Federative Republic of Brazil (includes Fernando de Noronha Island, Martim Vaz Islands, and BR Brazil Trindade Island) BS Bahamas Commonwealth of the Bahamas BT Bhutan Kingdom of Bhutan -

Argentine and Chilean Claims to British Antarctica. - Bases Established in the South Shetlands
Keesing's Record of World Events (formerly Keesing's Contemporary Archives), Volume VI-VII, February, 1948 Argentine, Chilean, British, Page 9133 © 1931-2006 Keesing's Worldwide, LLC - All Rights Reserved. Argentine and Chilean Claims to British Antarctica. - Bases established in the South Shetlands. - Chilean President inaugurates Chilean Army Bases on Greenwich Island. - Argentine Naval Demonstration in British Antarctic Waters. - H.M.S. "Nigeria" despatched to Falklands. - British Government Statements. - Argentine-Chilean Agreement on Joint Defence of "Antarctic Rights." - The Byrd and Ronne Antarctic Expeditions. - Australian Antarctic Expedition occupies Heard Islands. The Foreign-Office in London, in statements on Feb. 7 and Feb. 13, announced that Argentina and Chile had rejected British protests, earlier presented in Buenos Aires and Santiago, against the action of those countries in establishing bases in British Antarctic territories. The announcement of Feb. 7 stated that on Dec. 7, 1947, the British Ambassador in Buenos Aires, Sir Reginald Leeper, had presented a Note expressing British "anxiety" at the activities in the Antarctic of an Argentine naval expedition which had visited part of the Falkland Islands Dependencies, including Graham Land, the South Shetlands, and the South Orkneys, and had landed at various points in British territory; that a request had been made for Argentine nationals to evacuate bases established on Deception Island and Gamma Island, in the South Shetlands; that H.M. Government had proposed that the Argentine should submit her claim to Antarctic sovereignty to the International Court of Justice for adjudication; and that on Dec. 23, 1947, a second British Note had been presented expressing surprise at continued violations of British territory and territorial waters by Argentine vessels in the Antarctic. -

2017 All Countries of Citizenship by Total Number of Active Students
2017 All Countries of Citizenship by Total Number of Active SEVIS Records Country of Citizenship Total SEVIS IDs in 2017 CHINA 478,879 INDIA 247,133 REPUBLIC OF KOREA (SOUTH KOREA) 95,270 SAUDI ARABIA 72,084 JAPAN 41,534 CANADA 39,504 VIETNAM 37,955 BRAZIL 33,918 TAIWAN 32,372 MEXICO 23,539 NIGERIA 19,165 NEPAL 17,853 TURKEY 17,190 GERMANY 16,216 UNITED KINGDOM 15,774 COLOMBIA 15,390 VENEZUELA 15,286 THAILAND 14,609 FRANCE 14,447 IRAN 14,334 KUWAIT 13,477 SPAIN 11,481 INDONESIA 10,852 HONG KONG 10,224 ITALY 10,013 MALAYSIA 9,880 BANGLADESH 9,613 RUSSIA 9,309 PAKISTAN 8,883 AUSTRALIA 7,204 SWEDEN 6,331 SINGAPORE 6,144 PHILIPPINES 6,014 SWITZERLAND 5,118 PERU 4,886 ECUADOR 4,811 EGYPT 4,622 KENYA 4,447 NORWAY 4,234 NETHERLANDS 4,209 JAMAICA 4,194 CHILE 4,193 BAHAMAS, THE 4,118 GHANA 4,075 SRI LANKA 4,055 UKRAINE 3,975 2017 All Countries of Citizenship by Total Number of Active SEVIS Records MONGOLIA 3,904 JORDAN 3,874 OMAN 3,676 KAZAKHSTAN 3,599 ARGENTINA 3,532 ISRAEL 3,476 POLAND 3,358 UNITED ARAB EMIRATES 3,236 PANAMA 3,151 SOUTH AFRICA 3,001 GREECE 2,889 HONDURAS 2,830 ETHIOPIA 2,797 DENMARK 2,578 DOMINICAN REPUBLIC 2,431 MOROCCO 2,395 NEW ZEALAND 2,265 CAMEROON 2,172 BURMA 2,132 BELGIUM 2,125 LEBANON 2,031 SERBIA 2,028 QATAR 1,929 COTE D'IVOIRE 1,911 RWANDA 1,895 CONGO (KINSHASA) 1,889 ANGOLA 1,797 TRINIDAD AND TOBAGO 1,767 LIBYA 1,758 ZIMBABWE 1,751 BOLIVIA 1,713 EL SALVADOR 1,684 ROMANIA 1,622 GUATEMALA 1,606 COSTA RICA 1,557 AUSTRIA 1,536 IRELAND 1,530 PORTUGAL 1,514 CZECH REPUBLIC 1,388 BULGARIA 1,320 ALBANIA 1,298 TANZANIA 1,290 -
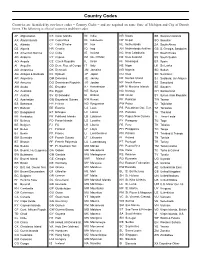
Two-Digit Country Codes for Tax Returns
Country Codes Countries are identified by two-letter codes – Country Codes – and are required on some State of Michigan and City of Detroit forms. The following is a list of countries and their codes. AF Afghanistan CK Cook Islands IN India NR Nauru SB Solomon Islands AX Åland Islands CR Costa Rica ID Indonesia NP Nepal SO Somalia AL Albania CI Côte D’ivoire IR Iran NL Netherlands ZA South Africa DZ Algeria HR Croatia IQ Iraq AN Netherlands Antilles GS S. Georgia, Sandwich AS American Samoa CU Cuba IE Ireland NC New Caledonia KR South Korea AD Andorra CY Cyprus IM Isle Of Man NZ New Zealand SS South Sudan AO Angola CZ Czech Republic IL Israel NI Nicaragua ES Spain AI Anguilla CD Dem. Rep. of Congo IT Italy NE Niger LK Sri Lanka AQ Antarctica DK Denmark JM Jamaica NG Nigeria SD Sudan AG Antigua & Barbuda DJ Djibouti JP Japan NU Niue SR Suriname AR Argentina DM Dominica JE Jersey NF Norfolk Island SJ Svalbard, Jan Mayen AM Armenia DO Dominican Republic JO Jordan KP North Korea SZ Swaziland AW Aruba EC Ecuador KZ Kazakhstan MP N. Mariana Islands SE Sweden AU Australia EG Egypt KE Kenya NO Norway CH Switzerland AT Austria SV El Salvador KI Kiribati OM Oman SY Syrian Arab Republic AZ Azerbaijan GQ Equatorial Guinea KW Kuwait PK Pakistan TW Taiwan BS Bahamas ER Eritrea KG Kyrgyzstan PW Palau TJ Tajikistan BH Bahrain EE Estonia LA Laos PS Palestinian Occ. Terr. TZ Tanzania BD Bangladesh ET Ethiopia LV Latvia PA Panama TH Thailand BB Barbados FK Falkland Islands LB Lebanon PG Papua New Guinea TL Timor-Leste BY Belarus FO Faroe Islands LS Lesotho PY Paraguay TG Togo BE Belgium FJ Fiji LR Liberia PE Peru TK Tokelau BZ Belize FI Finland LY Libya PH Philippines TO Tonga BJ Benin FR France LI Liechtenstein PN Pitcairn TT Trinidad & Tobago BM Bermuda GF French Guiana LT Lithuania PL Poland TN Tunisia BT Bhutan PF French Polynesia LU Luxembourg PT Portugal TR Turkey BO Bolivia TF Fr. -
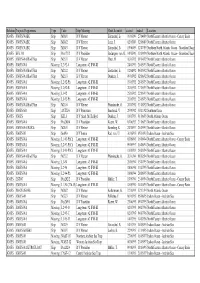
Relation Projects/Programmes Type Code Ship/Mooring Chief Scientist Started Ended Location JGOFS JGOFS/NABE Ship M10/1 R/V Meteor Zeitzschel, B
Relation Projects/Programmes Type Code Ship/Mooring Chief Scientist started ended Location JGOFS JGOFS/NABE Ship M10/1 R/V Meteor Zeitzschel, B. 19/03/89 27/04/89 North Eastern Atlantic Ocean - Canary Basin JGOFS JGOFS/NABE Ship M10/2 R/V Meteor Lenz, J. 02/05/89 12/06/89 North Eastern Atlantic Ocean JGOFS JGOFS/NABE Ship M10/3 R/V Meteor Zeitzschel, B. 15/06/89 12/07/89 Northern North Atlantic Ocean - Greenland Basin JGOFS SFB 313 Ship Pos173/2 R/V Poseidon Bodungen, von, B. 14/08/90 10/09/90 Northern North Atlantic Ocean - Greenland Basin JGOFS JGOFS-NA/BioCFlux Ship M21/1 R/V Meteor Thiel, H. 16/03/92 09/04/92 North Eastern Atlantic Ocean JGOFS JGOFS-NA Mooring L2-92-A Long-term #2 IFM-KI 24/03/92 26/05/93 North Eastern Atlantic Ocean JGOFS JGOFS-NA/BioCFlux Ship M21/2 R/V Meteor Zeitzschel, B. 12/04/92 06/05/92 North Eastern Atlantic Ocean JGOFS JGOFS-NA/BioCFlux Ship M21/3 R/V Meteor Duinker, J. 09/05/92 02/06/92 North Eastern Atlantic Ocean JGOFS JGOFS-NA Mooring L2-92-Ph Long-term #2 IFM-KI 19/05/92 28/05/93 North Eastern Atlantic Ocean JGOFS JGOFS-NA Mooring L2-92-B Long-term #2 IFM-KI 20/05/92 27/05/93 North Eastern Atlantic Ocean JGOFS JGOFS-NA Mooring L3-92 Long-term #3 IFM-KI 25/05/92 22/05/93 North Eastern Atlantic Ocean JGOFS JGOFS-NA Mooring L3-92-Ph Long-term #3 IFM-KI 26/05/92 23/05/93 North Eastern Atlantic Ocean JGOFS JGOFS-NA/BioCFlux Ship M21/6 R/V Meteor Pfannkuche, O. -

GENERAL AGREEMENT on L/253812January 1966 TARIFFS and TRADE Limited Distribution
RESTRICTED GENERAL AGREEMENT ON L/253812January 1966 TARIFFS AND TRADE Limited Distribution APPLICATION OF THEGENERALAGREEMENT Territories to which the Agreement is Applied Annexed hereto is a list of the contracting parties and of the territories (according to information available to the secretariat) in respect of which the application of the Agreement has been made effective. This list is a revision of that which appeared in the Twelfth Supplement, BISD pages 5-9. If there are any inaccuracies in this list, the contracting parties concerned are requested to notify the Director-General not later than 1March 1966 so that a revised list can be included in the Fourteenth Supplement which will be published after the twenty-third session. L/2538 Page 2 A. CONTRACTING PARTIES Australia, Commonwealth of Austria Belgium Brazil (including islands: Fernando de Noronha (including Rocks of Sao Pedro, Sao Paulo, Atoll das Rocas), Trinidad and Martim Vas) Burma Burundi Cameroon Canada Central African Republic Ceylon Chad Chile (including islands: Juan Fernandez group, Easter islands, Sala y Gomez, San Feliz, San Ambrosio and western part of Tierra del Fuego) Congo (Brazzaville) Caba, (including Isle of Pines and some smaller islands) Cyprus Czechoslovakia Dahomey Denmark (including the Färoe Islands) Dominican Republic (including islands: Saona, Catalina, Beata and some smaller ones) Finland France (including Corsica and islands off the French Coast and the Principality of Monaco) Comoro Archipelago (Grande-Comore, Anjouan, Mohély,Mayotte) French Guiana (including islands: St. Joseph, Iie Royale, Ilidu Diable) French Oceania (Society Islands, Ies Sous le Vent, MarquezesArchipelago, Tuamotu Archipelago, Gambior Archipelago, Tubuai Archipelago, Rapa island and Clipoerton Island) French Somaliland (including islands: Maskali and Mourba, Frères) Guadeloupe (islands of Gualoupe Basse-Terre and Grande-Terre and dependencies: Marie-Galante, Iledes Saintes, Petite-Teree, St.