The Pharyngeal Pouches and Clefts: Development, Evolution, Structure and Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endothelium in the Pharyngeal Arches 3, 4 and 6 Is Derived from the Second Heart Field
Thomas Jefferson University Jefferson Digital Commons Center for Translational Medicine Faculty Papers Center for Translational Medicine 1-15-2017 Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Xia Wang Thomas Jefferson University Dongying Chen Thomas Jefferson University Kelley Chen Thomas Jefferson University Ali Jubran Thomas Jefferson University AnnJosette Ramirez Thomas Jefferson University Follow this and additional works at: https://jdc.jefferson.edu/transmedfp Part of the Translational Medical Research Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Wang, Xia; Chen, Dongying; Chen, Kelley; Jubran, Ali; Ramirez, AnnJosette; and Astrof, Sophie, "Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field." (2017). Center for Translational Medicine Faculty Papers. Paper 45. https://jdc.jefferson.edu/transmedfp/45 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Center for Translational Medicine Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Authors Xia Wang, Dongying Chen, Kelley Chen, Ali Jubran, AnnJosette Ramirez, and Sophie Astrof This article is available at Jefferson Digital Commons: https://jdc.jefferson.edu/transmedfp/45 HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Dev Biol Manuscript Author . -

Embryology of Branchial Region
TRANSCRIPTIONS OF NARRATIONS FOR EMBRYOLOGY OF THE BRANCHIAL REGION Branchial Arch Development, slide 2 This is a very familiar picture - a median sagittal section of a four week embryo. I have actually done one thing correctly, I have eliminated the oropharyngeal membrane, which does disappear sometime during the fourth week of development. The cloacal membrane, as you know, doesn't disappear until the seventh week, and therefore it is still intact here, but unlabeled. But, I've labeled a couple of things not mentioned before. First of all, the most cranial part of the foregut, that is, the part that is cranial to the chest region, is called the pharynx. The part of the foregut in the chest region is called the esophagus; you probably knew that. And then, leading to the pharynx from the outside, is an ectodermal inpocketing, which is called the stomodeum. That originally led to the oropharyngeal membrane, but now that the oropharyngeal membrane is ruptured, the stomodeum is a pathway between the amniotic cavity and the lumen of the foregut. The stomodeum is going to become your oral cavity. Branchial Arch Development, slide 3 This is an actual picture of a four-week embryo. It's about 5mm crown-rump length. The stomodeum is labeled - that is the future oral cavity that leads to the pharynx through the ruptured oropharyngeal membrane. And I've also indicated these ridges separated by grooves that lie caudal to the stomodeum and cranial to the heart, which are called branchial arches. Now, if this is a four- week old embryo, clearly these things have developed during the fourth week, and I've never mentioned them before. -

Pharyngeal Arch Cheat Sheet V3
Pharyngeal Arches v3 (updates in red) Each pharyngeal arch contains: (1) a skeletal component (early on, a cartilaginous rod); (2) a muscular component; (3) an aortic arch; and (4) a nerve. Pharyngeal Arch Skeletal Component Muscles Aortic Arch Nerve First P. Arch Meckel's cartilage (malleus, incus) mm. of mastication, ant. belly 1st aortic arch Trigeminal N “mandibular maxillary prominence (maxilla*) of digastric, mylohyoid, tensor (maxillary AA) V2 and V3 arch” mandibular prominence (mandible*) tympani, tensor veli palatini Second P. Arch Reichert's cartilage (stapes, styloid mm. of facial expression, 2nd aortic arch Facial N “hyoid arch” process, stylohyoid lig.) buccinator, platysma, stapedius, (stapedial AA) VII hyoid, lesser cornua & superior body stylohyoid, post. belly of digastric Third P. Arch hyoid, greater cornua & inferior body stylopharyngeus 3rd aortic arch Glossophar. N (common carotids) IX Fourth P. Arch thyroid cartilage, epiglottic cartilage† cricothyroid, all intrinsic mm. 4th aortic arch Vagus: SLN of soft palate except tensor (aorta, R subclav.‡) & pharyn. plex. veli palatini X Fifth P. Arch - no derivatives - Sixth P. Arch cricoid, arytenoid, and corniculate all intrinsic mm. of larynx 6th aortic arch Vagus: RLN cartilages† except cricothyroid (pulmonary AA, X ductus arteriosus) * The maxillary and mandibular prominences are modeled on the first arch, but the bones develop separately from the first arch cartilage. † According to some sources the corniculate and cuneiform cartilages are derived from the 4th arch, not the 6th; I may revise this later on. ‡ Right subclavian A is derived from 4th aortic arch, right dorsal aorta, and right 7th intersegmental A; left subclavian A is derived from left 7th intersegmental A only, with no aortic arch component. -

The Development of Meckel's Cartilage in Staged Human Embryos During
Folia Morphol. Vol. 64, No. 1, pp. 23–28 Copyright © 2005 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl The development of Meckel’s cartilage in staged human embryos during the 5th week Maria Lorentowicz-Zagalak, Agnieszka Przystańska, Witold Woźniak Department of Anatomy, University School of Medical Sciences, Poznań, Poland [Received 30 December 2004; Accepted 1 Ferbruary 2005] The study was conducted on 15 embryos aged 5 weeks. The primordium of Meckel’s cartilage appears at stage 13 (32 days) as a rounded structure com- posed of fusiform and polygonal cells, which blend with other cells of the man- dibular process. At stages 14 and 15 (33 and 36 days) Meckel’s cartilage forms a well delineated core of small densely packed cells. Key words: human embryology, Meckel’s cartilage INTRODUCTION ossification. Some authors presume that Meckel’s The cartilaginous and skeletal elements of the cartilage has no relationship to ossification of the mandibular arch are formed from the embryonic mandible [20, 24, 26]. Lee et al. [19] found that the neural crest [14, 16, 17, 30, 33]. Recent studies in- intramembranous ossification and the condensed dicate that inductive epithelio-mesenchymal inter- cellular mesenchyme of the condylar blastema are actions mediated by diffusion factors are impor- closely associated with a portion of the perichon- tant during osteogenesis and odontogenesis with- dral fibrous tissue of Meckel’s cartilage. There are in the mandible [1, 3–5, 21–23, 31, 32, 34]. Of these also differences in the time of appearance and deg- factors a crucial role is played by epidermal growth radation of Meckel’s cartilage during the human in- factor (EGF), connective tissue growth factor tra-uterine period [2, 7, 9, 13, 15, 24, 27–30]. -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -
![The Pharyngeal Arches [PDF]](https://docslib.b-cdn.net/cover/8257/the-pharyngeal-arches-pdf-1328257.webp)
The Pharyngeal Arches [PDF]
24.3.2015 The Pharyngeal Arches Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow What is Pharyngeal Arch? • Rod-like thickenings of mesoderm present in the wall of the foregut. • They appear in 4th-5th weeks of development. • Contribute to the characteristic external appearance of the embryo. • As its development resembles with gills (branchia: Greek word) in fishes & amphibians, therefore also called as branchial arch. Formation of Pharyngeal Arches Lens N Pharyngeal Apparatus Pharyngeal apparatus consists of: • Pharyngeal arches • Pharyngeal pouches • Pharyngeal grooves/clefts • Pharyngeal membrane Pharyngeal Arches • Pharyngeal arches begin to develop early in the fourth week as neural crest cells migrate into the head and neck region. • The first pair of pharyngeal arches (primordium of jaws) appears as a surface elevations lateral to the developing pharynx. • Soon other arches appear as obliquely disposed, rounded ridges on each side of the future head and neck regions. Le N Pharyngeal Arches • By the end of the fourth week, four pairs of pharyngeal arches are visible externally. • The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo. • The pharyngeal arches are separated from each other by fissures called pharyngeal grooves/clefts. • They are numbered in craniocaudal sequence. Pharyngeal Arch Components • Each pharyngeal arch consists of a core of mesenchyme. • Is covered externally by ectoderm and internally by endoderm. • In the third week, the original mesenchyme is derived from mesoderm. • During the fourth week, most of the mesenchyme is derived from neural crest cells that migrate into the pharyngeal arches. Structures in a Pharyngeal Arch Arrangement of nerves supplying the pharyngeal arch (in lower animals) Fate of Pharyngeal Arches A typical pharyngeal arch contains: • An aortic arch, an artery that arises from the truncus arteriosus of the primordial heart. -

Development Of, Tongue, Thyroid, Sinus and Salivary Glands
Development of, tongue, thyroid, sinus and salivary glands Development of tongue • 1st ,2nd, 3rd, 4th pharyngeal arches • Median swelling- tuberculum impar • Two lateral swellings –lingual • Caudal medial swelling- hypobrachial eminence Anterior 2/3 of the tongue: • Formation: median and lateral tongue buds that arise from the floor of the 1st pharyngeal arch and then grow rostrally. • thus it is formed by fusion of -- • tuberculum impar , • two lingual swellings • The tongue buds are then invaded by occipital myoblasts that form the intrinsic muscles of the tongue. • Thus anterior 2/3rd of tonguer is supplied by lingual branch of mandibular nerve ,(post trematic nerve of this arch) and chorda tympani nerve( pretrematic nerve of arch) • posterior 1/3rd of tongue is supplied by glossopharyngeal nerve ( nerve of 3rd arch) • Most posterior 1/3rd of tongue is supplied by superior laryngeal nerve ( nerve of 4th arch) • Musculature of tongue is derived from occipital myotomes --explains nerve supply by hypoglossal nerve, nerve of these myotomes. Posterior 1/3rd of tongue • formed from cranial part of hypobranchial eminence ( copula) • the second arch mesoderm gets buried below the surface . • the third arch mesoderm grows over it to fuse with mesoderm of first arch . • posterior one third of tongue thus formed by third arch mesoderm. • posterior most part of tongue is derived from fourth arch • Thus swellings from the floor of the 3rd and 4th pharyngeal arches overgrow the 2nd arch and fuse with the anterior 2/3 of the tongue. • posterior 1/3 of the tongue is derived from the 3rd and 4th arches • Intrinsic musculature is also derived from occipital myoblasts. -

Aortic Arches - Embryological Development
Aortic Arches - Embryological Development Aortic Arches These embryonic structures form during the development of the arterial system in intrauterine life. An aortic arch is a branch from the arterial aortic sac to the dorsal aorta. It travels in the centre of each pharyngeal arch, embedded in mesenchyme. Initially there are five pairs of arches, but these undergo structural changes and differentiation to form the definite vascular patterns for the head and neck, aorta, and pulmonary circulation. During the fourth and fifth weeks of embryological development, when the pharyngeal arches form, the aortic sac gives rise to arteries – the aortic arches. The aortic sac is the endothelial lined dilation just distal to the truncus arteriosus; it is the primordial vascular channel from which the aortic arches arise. Each pharyngeal arch has its own cranial nerve and its own artery, hence we can conclude that the growth of the the aortic and pharyngeal arches are very closely related. The aortic arches terminate in the right and left dorsal aortae. The dorsal aortae remain paired in the region of the arches, however below this region they merge to form a single vessel (the descending/thoracic/abdominal aorta). The pharyngeal arches and their vessels appear in a cephalo-caudal order, so they are not all present at the same time. As a new arch forms the aortic sac contributes a branch to it. In the initial stage there are pairs of aortic arches, which are numbered I, II, III, IV, and V. This system becomes altered in further development. The truncus arteriosus divides into the ventral aorta and pulmonary trunk by the aortic-pulmonary septum. -
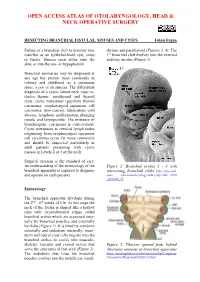
Resecting Branchial Cysts, Fistulae and Sinuses
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY RESECTING BRANCHIAL FISTULAE, SINUSES AND CYSTS Johan Fagan Failure of a branchial cleft to involute may thymus and parathyroid (Figures 3, 4). The manifest as an epithelial-lined cyst, sinus 1st branchial cleft evolves into the external or fistula. Sinuses open either onto the auditory meatus (Figure 3). skin, or into the oro- or hypopharynx. Branchial anomalies may be diagnosed at any age but present most commonly in infancy and childhood as a cutaneous sinus, a cyst or an abscess. The differential diagnosis of a cystic lateral neck mass in- cludes thymic, parathyroid and thyroid cysts, cystic metastases (papillary thyroid carcinoma, oropharyngeal squamous cell carcinoma, skin cancer), tuberculous cold abscess, lymphatic malformation, plunging ranula, and laryngocoele. The existence of branchiogenic carcinoma is controversial. Cystic metastases to cervical lymph nodes originating from oropharyngeal squamous cell carcinoma occur far more commonly and should be suspected particularly in adult patients presenting with cystic masses in Levels 2 or 3 of the neck. Surgical excision is the standard of care. An understanding of the embryology of the Figure 1: Branchial arches 1 - 4 with branchial apparatus is required to diagnose intervening branchial clefts http://php.med. and operate on such patients. unsw edu.au/embryology/index.php?title=2010 _Lecture_11 Embryology The branchial apparatus develops during the 2nd - 6th weeks of life. At this stage the neck of the foetus is shaped like a hollow tube with circumferential ridges called branchial arches which are separated inter- nally by branchial pouches and externally by clefts (Figure 1). -

Cervical Cysts and Fistulae
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1938 Cervical cysts and fistulae Frederick Dee Koehne University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Koehne, Frederick Dee, "Cervical cysts and fistulae" (1938). MD Theses. 673. https://digitalcommons.unmc.edu/mdtheses/673 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. ,;/'"- CERVICAL CYSTS AND F'ISTULAE FREDERICK DEE KOEHNE Senior Thesis Presented to the University of Nebraska College of Medicine, Omaha, 1938. / -- / OUTLINE I. Introduction. A. In~oductory statement B. Definition c. Classification 1. Lateral cysts and fistulae 2. Mid-line cysts and fistulae D. Purpose II. Hlsto191' III. Lateral or BM.. nchial Cysts and Fistulae. A. Embryology 1. Normal embryology of the branchial ap parat_us. 2. Cervical Sinus theory--Second branchial cleft. 3. Thymic duct theorr• 4. Any cleft and cervical sinus. B. Pathology C. · S}'Dlptoma , , diagnosis and di ff eren tial diagnosis. D. Treatment~ IV. Mid-line or Thyroglossal duct cysts and fistulae. A. Embryology 1. Normal 2. Theory -~s to origin. B. Pathology 480951 c. Symptoms, di"agnosis and differential diagnosis. D. Treatment. v • sUlllil18.17' • r 1. PAR'r I. Introduction. The subject of Cervical cysts and fistuae has, for the past century, been one for considerable speculation and controversy. -

Embryology and Teratology in the Curricula of Healthcare Courses
ANATOMICAL EDUCATION Eur. J. Anat. 21 (1): 77-91 (2017) Embryology and Teratology in the Curricula of Healthcare Courses Bernard J. Moxham 1, Hana Brichova 2, Elpida Emmanouil-Nikoloussi 3, Andy R.M. Chirculescu 4 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3AX, Wales, United Kingdom and Department of Anatomy, St. George’s University, St George, Grenada, 2First Faculty of Medicine, Institute of Histology and Embryology, Charles University Prague, Albertov 4, 128 01 Prague 2, Czech Republic and Second Medical Facul- ty, Institute of Histology and Embryology, Charles University Prague, V Úvalu 84, 150 00 Prague 5 , Czech Republic, 3The School of Medicine, European University Cyprus, 6 Diogenous str, 2404 Engomi, P.O.Box 22006, 1516 Nicosia, Cyprus , 4Department of Morphological Sciences, Division of Anatomy, Faculty of Medicine, C. Davila University, Bucharest, Romania SUMMARY Key words: Anatomy – Embryology – Education – Syllabus – Medical – Dental – Healthcare Significant changes are occurring worldwide in courses for healthcare studies, including medicine INTRODUCTION and dentistry. Critical evaluation of the place, tim- ing, and content of components that can be collec- Embryology is a sub-discipline of developmental tively grouped as the anatomical sciences has biology that relates to life before birth. Teratology however yet to be adequately undertaken. Surveys (τέρατος (teratos) meaning ‘monster’ or ‘marvel’) of teaching hours for embryology in US and UK relates to abnormal development and congenital medical courses clearly demonstrate that a dra- abnormalities (i.e. morphofunctional impairments). matic decline in the importance of the subject is in Embryological studies are concerned essentially progress, in terms of both a decrease in the num- with the laws and mechanisms associated with ber of hours allocated within the medical course normal development (ontogenesis) from the stage and in relation to changes in pedagogic methodol- of the ovum until parturition and the end of intra- ogies. -

Arches 1 and 2 Arch 3 Arch 4 Arch 5 Arch 6
SEM IV Study Materials AORTIC ARCHES INTRODUCTION The aortic arches or pharyngeal arch arteries (previously referred to as branchial arches in human embryos) are a series of six paired embryological vascular structures which give rise to the great arteries of the neck and head. They are ventral to the dorsal aorta and arise from the aortic sac. The aortic arches are formed sequentially within the pharyngeal arches and initially appear symmetrical on both sides of the embryo,[1] but then undergo a significant remodelling to form the final asymmetrical structure of the great arteries. Structure: Arches 1 and 2 The first and second arches disappear early. A remnant of the 1st arch forms part of the maxillary artery, a branch of the external carotid artery. The ventral end of the second develops into the ascending pharyngeal artery, and its dorsal end gives origin to the stapedial artery, a vessel which typically atrophies in humans but persists in some mammals. The stapedial artery passes through the ring of the stapes and divides into supraorbital, infraorbital, and mandibular branches which follow the three divisions of the trigeminal nerve. The infraorbital and mandibular branches arise from a common stem, the terminal part of which anastomoses with the external carotid artery. On the obliteration of the stapedial artery, this anastomosis enlarges and forms the internal maxillary artery; branches formerly of the stapedial artery are subsequently considered branches of the internal maxillary artery. The common stem of the infraorbital and mandibular branches passes between the two roots of the auriculotemporal nerve and becomes the middle meningeal artery; the original supraorbital branch of the stapedial is represented by the orbital twigs of the middle meningeal.