Switch List Updated June 21, 2021.Doc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

MSM Cross Reference Antihistamine Decongestant 20100701 Final Posted
MISSISSIPPI DIVISION OF MEDICAID Antihistamine/Decongestant Product and Active Ingredient Cross-Reference List The agents listed below are the antihistamine/decongestant drug products listed in the Mississippi Medicaid Preferred Drug List (PDL). This is a cross-reference between the drug product name and its active ingredients to reference the antihistamine/decongestant portion of the PDL. For more information concerning the PDL, including non- preferred agents, the OTC formulary, and other specifics, please visit our website at www.medicaid.ms.gov. List Effective 07/16/10 Therapeutic Class Active Ingredients Preferred Non-Preferred ANTIHISTAMINES - 1ST GENERATION BROMPHENIRAMINE MALEATE BPM BROMAX BROMPHENIRAMINE MALEATE J-TAN PD BROMSPIRO LODRANE 24 LOHIST 12HR VAZOL BROMPHENIRAMINE TANNATE BROMPHENIRAMINE TANNATE J-TAN P-TEX BROMPHENIRAMINE/DIPHENHYDRAM ALA-HIST CARBINOXAMINE MALEATE CARBINOXAMINE MALEATE PALGIC CHLORPHENIRAMINE MALEATE CHLORPHENIRAMINE MALEATE CPM 12 CHLORPHENIRAMINE TANNATE ED CHLORPED ED-CHLOR-TAN MYCI CHLOR-TAN MYCI CHLORPED PEDIAPHYL TANAHIST-PD CLEMASTINE FUMARATE CLEMASTINE FUMARATE CYPROHEPTADINE HCL CYPROHEPTADINE HCL DEXCHLORPHENIRAMINE MALEATE DEXCHLORPHENIRAMINE MALEATE DIPHENHYDRAMINE HCL ALLERGY MEDICINE ALLERGY RELIEF BANOPHEN BENADRYL BENADRYL ALLERGY CHILDREN'S ALLERGY CHILDREN'S COLD & ALLERGY COMPLETE ALLERGY DIPHEDRYL DIPHENDRYL DIPHENHIST DIPHENHYDRAMINE HCL DYTUSS GENAHIST HYDRAMINE MEDI-PHEDRYL PHARBEDRYL Q-DRYL QUENALIN SILADRYL SILPHEN DIPHENHYDRAMINE TANNATE DIPHENMAX DOXYLAMINE SUCCINATE -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

HMSA Drug Formulary
March 23, 2004 MEMORANDUM TO: Participating Pharmacies FROM: John T. Berthiaume, M.D. Medical Director, Pharmacy Management SUBJECT: Updated HMSA Drug Formulary Enclosed is the comprehensive updated formulary, effective April 1. This copy incorporates the changes listed in the Formulary Update sent to you in February. Please replace the current formulary sections in your pharmacy handbook with the enclosed version. If you have any questions, please call an HMSA Provider Teleservice Representative at 948- 6330 on Oahu or 1 (800) 790-4672 from the Neighbor Islands. PM04-010 HMSA DRUG FORMULARY 4/1/04 - Page 1 Code THERAPEUTIC CATEGORY LISTING 1 I. Anti-infectives A. Antibiotics 1. Penicillins - non-penicillinase resistant 2 I. Anti-infectives A. Antibiotics 2. Penicillins - penicillinase resistant 3 I. Anti-infectives A. Antibiotics 3. Cephalosporins 4 I. Anti-infectives A. Antibiotics 4. Fluoroquinolones 5 I. Anti-infectives A. Antibiotics 5. Tetracyclines 6 I. Anti-infectives A. Antibiotics 6. Macrolides 7 I. Anti-infectives A. Antibiotics 7. Vancomycin 8 I. Anti-infectives A. Antibiotics 8. Lincosamides 9 I. Anti-infectives A. Antibiotics 9. Aminoglycoside 10 I. Anti-infectives A. Antibiotics 10. Sulfonamides 11 I. Anti-Infectives A. Antibiotics 11. Vaginal preparations 12 I. Anti-infectives B. Antifungal agents 1. Oral 13 I. Anti-Infectives B. Antifungal agents 2. Vaginal preparations OTC considerations: clotrimazole (Gyne-Lotrimin 3, Mycelex-7), miconazole (Monistat 7), tioconazole (Vagistat-1), butoconazole (Femstat 3) 14 I. Anti-infectives C. Antimalarial 15 I. Anti-infectives D. Antituberculous 16 I. Anti-infectives E. Amebicides 17 I. Anti-infectives F. Antiviral agents 1. Nucleoside Reverse-transcriptase Inhibitors (NRTI) 18 I. -

Inhibition of Histamine-Induced Human Conjunctival Epithelial Cell Responses by Ocular Allergy Drugs
LABORATORY SCIENCES Inhibition of Histamine-Induced Human Conjunctival Epithelial Cell Responses by Ocular Allergy Drugs John M. Yanni, PhD; Lori K. Weimer, BA; Najam A. Sharif, PhD; Shou X. Xu, MS; Daniel A. Gamache, PhD; Joan M. Spellman, MS Objective: To evaluate the effects of topical ocular drugs secretion (50% inhibitory concentration, 1.7-5.5 nmol/L) with histamine H1-antagonist activity on histamine- than predicted from binding data, while antazoline and stimulated phosphatidylinositol turnover and interleu- pheniramine were far less potent (20- to 140-fold) in func- kin (IL) 6 and IL-8 secretion from human conjunctival tional assays. Levocabastine (dissociation constant, 52.6 epithelial cells. nmol/L) exhibited greater functional activity (50% in- hibitory concentration, 8-25 nmol/L) than either antazo- Methods: Primary human conjunctival epithelial cell cul- line or pheniramine. tures were stimulated with histamine in the presence or ab- sence of test drugs. Phosphatidylinositol turnover was quan- Conclusions: Histamine-stimulated phosphatidylino- tified by ion exchange chromatography and cytokine con- sitol turnover and cytokine secretion by human conjunc- tentofsupernatantsbyenzyme-linkedimmunosorbentassay. tival epithelial cells are attenuated by compounds with H1-antagonist activity. However, antihistaminic po- Results: Antazoline hydrochloride, emedastine difuma- tency alone does not predict anti-inflammatory poten- rate, levocabastine hydrochloride, olopatadine hydro- tial. Olopatadine, emedastine, and levocabastine were no- chloride, and pheniramine maleate attenuated histamine- tably more potent than pheniramine and antazoline. stimulated phosphatidylinositol turnover and IL-6 and IL-8 secretion. Emedastine was the most potent in li- Clinical Relevance: Selected topical ocular drugs with gand binding, phosphatidylinositol turnover, and IL-6 se- antihistaminic activity may offer therapeutic advan- cretion, with dissociation constant and 50% inhibitory tages to patients with allergic conjunctivitis by inhibit- concentrations of 1-3 nmol/L. -

Medication Other Information Aches and Pains Constipation Cough/Cold Diarrhea Fever Over the Counter Medications in Pregnancy
Over the Counter Medications in Pregnancy Women commonly use over the counter medications in pregnancy. This is a list of those medication you may safely use during pregnancy. If you have any questions about these medications and how to use them please contact your Healthcare Provider's office. Unless otherwise stated please take the medication as directed on the manufactures label. Medication Other Information Aches and Pains * Tylenol Extra Strength 500 mg tablet No more than 6 tablets in a 24 hour period * Tylenol 325 mg tablet No more than 8 in a 24 hour period Constipation Stool Softener Increases the amount of water in your stools to make them easier to pass * Colace (docusate sodium) * Surfak (docusate calcium) * Docusate Fiber Laxative Increases the amount of bulk in your stools to make them easier to pass * Metamucil (psyllium) * Fibercon (Calcium polycarbophil 625 mg) Stool Softener/Fiber Laxative Increases the amount of water in your stools to make them easier to pass * Peri-Colace (docusate sodium/sennosides) * Senekot -S (docusate sodium/sennosides) Osmotic Laxative Increases the amount of water in your stools to make them easier to pass * Milk of Magnesia * MiraLax (polyethylene glycol 3350) Cough/Cold Expectorants Help thin mucus and phlegm so they can be coughed up * Robitussin * Guaifenesin Antihistamines Can be used to relieve seasonal allergy and common cold symptoms of nasal congestion, * Chlorpheniramine sneezing and itchy eyes Cough Suppressants Help calm a cough * Dextromethorphan Decongestants Are used to relieve -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Medication Instructions for Allergy Patients
MEDICATION INSTRUCTIONS FOR ALLERGY PATIENTS Drugs which contain antihistamine or have antihistaminic effects can result in negative reactions to skin testing. As a result, it may not be possible to properly interpret skin test results, and testing may have to be repeated at a later date. While this list is extensive, it is NOT all inclusive (particularly of the various brand names). Discontinue ALL antihistamines including the following medications seven (7) days prior to skin testing (unless longer time specified): Antihistamines – Generic name (Brand name(s)): Cetirizine (Zyrtec, Zyrtec-D) Hydroxyzine (Vistaril, Atarax) Desloratadine (Clarinex) Levocetirizine (Xyzal) Fexofenadine (Allegra, Allegra-D) Loratadine (Claritin, Claritin-D, Alavert) Diphenhydramine (Aleve PM, Benadryl, Bayer P.M., Benylin, Contac P.M., Doans P.M, Excedrin PM, Legatrin P.M.. Nytol, Tylenol Nighttime, Unisom, Zzzquil) Chlorpheniramine (Aller-Chlor, Allerest, Alka Seltzer Plus, Chlor-Trimeton, Comtrex, Contac, Co-Pyronil, Coricidin, CTM, Deconamine, Dristan, Dura-tap, Naldecon, Ornade Spansules, Rondec, Sinutab, Teldrin, Triaminic, Triaminicin, Tylenol Allergy) Azatadine (Optimine, Trinalin) Doxylamine (Nyquil) Brompheniramine (Bromfed, Dimetane, Dimetapp) Meclizine (Antivert) Carbinoxamine (Clistin, Rondec) Pheniramine Clemastine (Tavist) Phenyltoloxamine (Nadecon) Cyclizine (Marezine) Promethazine (Phenergan) Cyprohepatidine (Periactin) (9 days) Pyrilamine (Mepyramine) Dexbrompheniramine (Drixoral) Quinacrine (Atabrine) Dexchlorpheniramine (Extendryl, Polaramine) -

Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
PUBLIC COPY Notice under s66 of the Commerce Act 1986 Application by Johnson & Johnson to acquire the stock, assets and business of the Consumer Healthcare division of Pfizer Inc. COMMERCE ACT 1986: BUSINESS ACQUISITION SECTION 66: NOTICE SEEKING CLEARANCE 28 September 2006 The Registrar Business Acquisitions and Authorisations Commerce Commission PO Box 2351 Wellington Pursuant to s66(1) of the Commerce Act 1986 notice is hereby given seeking clearance of a proposed business acquisition. 518689_1.DOC 2 CONTENTS EXECUTIVE SUMMARY PART 1: TRANSACTION DETAILS 1 The business acquisition for which clearance is sought 2 The person giving this notice 3 Confidentiality 4 Participants 5 Interconnected and associated persons 6 Beneficial interests 7 Links between participants 8 Common directorships 9 Business activities of the participants 10 Reasons for the proposed acquisition PART II: IDENTIFICATION OF MARKETS AFFECTED 11 Horizontal aggregation 12 Differentiated product markets 13 Differentiated product markets 14 Vertical integration 15 Other business acquisitions PARTS III, IV AND V: CONSTRAINTS ON MARKET POWERS BY EXISTING AND POTENTIAL COMPETITION AND OTHER POTENTIAL CONSTRAINTS 16 Allergy medication 17 Products for the treatment of worms 18 Thrush treatment CERTIFICATE APPENDICES 1. Heartburn and indigestion remedies (MYLANTA and MOTILIUM) 2. Worm treatments (COMBANTRIN, VERMOX) 3. Cold, flu, nasal decongestant, cough relief and sort throat medications (CODRAL, SINUTAB, SUDAFED, BENADRYL, BRONDECON) 4. Allergy relief products (ACTIFED, SINUTAB, SUDAFED, VISINE, LIVOSTIN) 518689_1.DOC 3 5. Thrush treatments (DIFLUCAN ONE, DAKTARIN, DAKTAGOLD, NIZORAL, SPORANOX) 6. Shampoo (PREGAINE, ROGAINE, NEUTROGENA, JOHNSON’S BABY SHAMPOO) 7. Hand hygiene (PURELL and MICRO SHIELD) 8. Competitor worm treatment products 9. Multinational pharmaceutical businesses: GlaxoSmithKline, Douglas Pharmaceuticals, Alphapharm, Bayer Group 10. -

Determination of Antidepressants in Human Plasma by Modified Cloud
pharmaceuticals Article Determination of Antidepressants in Human Plasma by Modified Cloud-Point Extraction Coupled with Mass Spectrometry El˙zbietaGniazdowska 1,2 , Natalia Korytowska 3 , Grzegorz Kłudka 3 and Joanna Giebułtowicz 3,* 1 Łukasiewicz Research Network, Industrial Chemistry Institute, 8 Rydygiera, 01-793 Warsaw, Poland; [email protected] 2 Department of Bioanalysis and Drugs Analysis, Doctoral School, Medical University of Warsaw, 61 Zwirki˙ i Wigury, 02-091 Warsaw, Poland 3 Department of Bioanalysis and Drugs Analysis, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha, 02-097 Warsaw, Poland; [email protected] (N.K.); [email protected] (G.K.) * Correspondence: [email protected] Received: 5 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Cloud-point extraction (CPE) is rarely combined with liquid chromatography coupled to mass spectrometry (LC–MS) in drug determination due to the matrix effect (ME). However, we have recently shown that ME is not a limiting factor in CPE. Low extraction efficiency may be improved by salt addition, but none of the salts used in CPE are suitable for LC–MS. It is the first time that the influences of a volatile salt—ammonium acetate (AA)—on the CPE extraction efficiency and ME have been studied. Our modification of CPE included also the use of ethanol instead of acetonitrile to reduce the sample viscosity and make the method more environmentally friendly. We developed and validated CPE–LC–MS for the simultaneous determination of 21 antidepressants in plasma that can be useful for clinical and forensic toxicology. The selected parameters included Triton X-114 concentration (1.5 and 6%, w/v), concentration of AA (0, 10, 20 and 30%, w/v), and pH (3.5, 6.8 and 10.2). -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
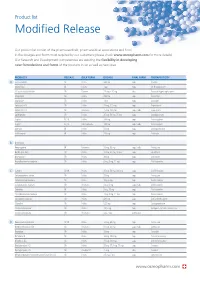
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine