Early Developmental Alterations in Gabaergic Protein Expression in Fragile X Knockout Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
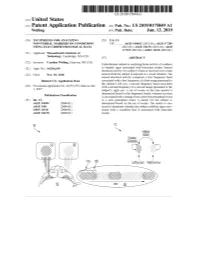
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

Effect of Repeated Gaboxadol Administration on Night Sleep and Next-Day Performance in Healthy Elderly Subjects
Neuropsychopharmacology (2005) 30, 833–841 & 2005 Nature Publishing Group All rights reserved 0893-133X/05 $30.00 www.neuropsychopharmacology.org Effect of Repeated Gaboxadol Administration on Night Sleep and Next-Day Performance in Healthy Elderly Subjects 1 2 ,3 1 Stefan Mathias , Josef Zihl , Axel Steiger* and Marike Lancel 1Section of Sleep Pharmacology, Max-Planck-Institute of Psychiatry, Munich, Germany; 2Section of Neuropsychology, Max-Planck-Institute of Psychiatry, Munich, Germany; 3Department of Psychiatry, Max-Planck-Institute of Psychiatry, Munich, Germany Aging is associated with dramatic reductions in sleep continuity and sleep intensity. Since gaboxadol, a selective GABAA receptor agonist, has been demonstrated to improve sleep consolidation and promote deep sleep, it may be an effective hypnotic, particularly for elderly patients with insomnia. In the present study, we investigated the effects of subchronic gaboxadol administration on nocturnal sleep and its residual effects during the next days in elderly subjects. This was a randomized, double-blind, placebo-controlled, balanced crossover study in 10 healthy elderly subjects without sleep complaints. The subjects were administered either placebo or 15 mg gaboxadol hydrochloride at bedtime on three consecutive nights. Sleep was recorded during each night from 2300 to 0700 h and tests assessing attention (target detection, stroop test) and memory function (visual form recognition, immediate word recall, digit span) were applied at 0900, 1400, and 1700 h during the following days. Compared with placebo, gaboxadol significantly shortened subjective sleep onset latency and increased self-rated sleep intensity and quality. Polysomnographic recordings showed that it significantly decreased the number of awakenings, the amount of intermittent wakefulness, and stage 1, and increased slow wave sleep and stage 2. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome
ORIGINAL RESEARCH published: 25 June 2019 doi: 10.3389/fnbeh.2019.00141 Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome Patricia Cogram 1,2,3,4, Robert M. J. Deacon 1,2,3,4, Jennifer L. Warner-Schmidt 5, Melanie J. von Schimmelmann 6, Brett S. Abrahams 6,7 and Matthew J. During 6,8* 1FRAXA-DVI, FRAXA Research Foundation, Boston, MA, United States, 2Centre for Systems Biotechnology, Biomedicine Division, Fraunhofer-Gesellschaft, Santiago, Chile, 3GEN.DDI Limited, London, United Kingdom, 4Institute of Ecology and Biodiversity (IEB), University of Chile, Santiago, Chile, 5NeuroJenic Consulting, LLC, Garden City, NY, United States, 6Ovid Therapeutics, New York, NY, United States, 7Department of Genetics and Neuroscience, Albert Einstein College of Medicine, Bronx, NY, United States, 8Department of Neurological Surgery and Molecular Virology, Immunology and Medical Genetics, Ohio State University College of Medicine, Columbus, OH, United States Fragile X syndrome (FXS) is the most common inherited form of intellectual disability and autism. FXS is also accompanied by attention problems, hyperactivity, anxiety, aggression, poor sleep, repetitive behaviors, and self-injury. Recent work supports the role of g-aminobutyric-acid (GABA), the primary inhibitory neurotransmitter in the brain, in mediating symptoms of FXS. Deficits in GABA machinery have been observed in a mouse model of FXS, including a loss of tonic inhibition in the amygdala, which is Edited by: Martine Ammassari-Teule, mediated by extrasynaptic GABAA receptors. Humans with FXS also show reduced Italian National Research Council GABAA receptor availability. Here, we sought to evaluate the potential of gaboxadol (CNR), Italy (also called OV101 and THIP), a selective and potent agonist for delta-subunit-containing Reviewed by: extrasynaptic GABA receptors (dSEGA), as a therapeutic agent for FXS by assessing Giulia Poggi, A University of Zurich, Switzerland its ability to normalize aberrant behaviors in a relatively uncharacterized mouse model Valerie J. -

Ligand-Gated Ion Channels' British Journal of Pharmacology, Vol
Edinburgh Research Explorer The Concise Guide to PHARMACOLOGY 2015/16 Citation for published version: Alexander, SP, Peters, JA, Kelly, E, Marrion, N, Benson, HE, Faccenda, E, Pawson, AJ, Sharman, JL, Southan, C, Davies, JA & CGTP Collaborators 2015, 'The Concise Guide to PHARMACOLOGY 2015/16: Ligand-gated ion channels' British Journal of Pharmacology, vol. 172, no. 24, pp. 5870-5903. DOI: 10.1111/bph.13350 Digital Object Identifier (DOI): 10.1111/bph.13350 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: British Journal of Pharmacology General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Apr. 2019 S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: Ligand-gated ion channels. British Journal of Pharmacology (2015) 172, 5870–5903 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: Ligand-gated ion channels Stephen PH Alexander1, -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

ICADTS \(28-30 Août 2007, Seattle, USA\)
Annales de Toxicologie Analytique, vol. XIX, n° 4, 2007 ORAL PRESENTATIONS RESULTS: There were 7,673 external cause deaths in Victoria between July 2000 and June 2005. Of these, COMMUNICATIONS ORALES there were 2,086 (27%) external cause deaths contained a toxicology report where alcohol was identified as positive (greater than 0). A toxicology report was not Postmortem toxicology attached in 1,420 (18.5%) cases and the remaining Toxicologie postmortem 4,152 external cause cases contained a negative result for alcohol. Of cases with a toxicology report 455 Understanding forensic toxicology in (5.4%) had a blood alcohol concentration (BAC) of less than 0.06 g/100 mL. There were 564 (7.4%) detected relation to external-cause deaths with alcohol (0.06 g/100mL to <0.16 g/100mL) and 659 J. KILLIAN(1), O. DRUMMER(2), J. OZANNE- (8.6%) had a toxic level of alcohol (>0.16 g/100 mL). SMITH(1) Of all causes with alcohol detected, 2726 (37%) were (1) Monash University Accident Research Centre, due to intentional self-harm, followed by 2005 (27%) being transport related, 932 (12%) due to poisoning and Victoria, Australia 553 (7%) being fall related. The trends and themes of (2) Monash University Department of Forensic other drugs will also be reported especially in relation Medicine and Victorian Institute of Forensic Medicine, to traffic injuries. Victoria, Australia CONCLUSIONS: The study results provide, for the AIMS: Injuries are not only recognised as an important first time in Australia, a systematic examination of the public health problem, but are also one of the major epidemiology of licit and illicit drugs in injury deaths causes of death. -

GABA Site Agonist Gaboxadol Induces Addiction-Predicting Persistent Changes in Ventral Tegmental Area Dopamine Neurons but Is Not Rewarding in Mice Or Baboons
5310 • The Journal of Neuroscience, April 11, 2012 • 32(15):5310–5320 Behavioral/Systems/Cognitive GABA Site Agonist Gaboxadol Induces Addiction-Predicting Persistent Changes in Ventral Tegmental Area Dopamine Neurons But Is Not Rewarding in Mice or Baboons Elena Vashchinkina,1 Anne Panhelainen,1 Olga Yu. Vekovischeva,1 Teemu Aitta-aho,1 Bjarke Ebert,2 Nancy A. Ator,3 and Esa R. Korpi1 1Institute of Biomedicine, Pharmacology, University of Helsinki, FI-00014 Helsinki, Finland, 2H. Lundbeck A/S, DK-2500 Copenhagen, Denmark, and 3Division of Behavioral Biology, Psychiatry, and Behavioral Sciences, The Johns Hopkins School of Medicine, Baltimore, Maryland 21224-6823 Dopamine neurons of the ventral tegmental area (VTA) are involved at early phases of drug addiction. Even the first in vivo dose of various abused drugs induces glutamate receptor plasticity at the excitatory synapses of these neurons. Benzodiazepines that suppress the inhibitory GABAergic interneurons in the VTA via facilitation of synaptic GABAA receptors have induced neuroplas- ticity in dopamine neurons due to this disinhibitory mechanism. Here, we have tested a non-benzodiazepine direct GABA site agonist 4,5,6,7-tetrahydroisoxazolol[4,5-c]pyridine-3-ol (THIP) (also known as gaboxadol) that acts preferentially via high- affinity extrasynaptic GABAA receptors. A single sedative dose of THIP (6 mg/kg) to mice induced glutamate receptor plasticity for at least 6 d after administration. Increased AMPA/NMDA receptor current ratio and increased frequency, amplitude, and rectifi- cation of AMPA receptor responses suggested persistent targeting of GluA2-lacking AMPA receptors in excitatory synapses of VTA ␦Ϫ/Ϫ dopamine neurons ex vivo after THIP administration. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

H J2G3 (58) (685-686)
WIADOMOŚCI h J2G3 3 > Z , 'b 2004 (58) 7-8 (685-686) CZASOPISMO POLSKIEGO TOWARZYSTWA CHEMICZNEGO Publikacja dotowana przez KBN RADA REDAKCYJNA RYSZARD ADAMIAK, JERZY BŁAŻEJOWSKI, JÓZEF CEYNOWA, JACEK GAWROŃSKI, JACEK KIJEŃSKI, TADEUSZ M. KRYGOWSKI, JACEK MŁOCHOWSKI. PIOTR PANETH, LEONARD M. PRONIEWICZ, WŁADYSŁAW RUDZIŃSKI, STANISŁAW SŁOMKOWSKI, JAN ZAWADIAK Z REDAKCJĄ STALE WSPÓŁPRACUJĄ HENRYK GALINA (Rzeszów), MAREK K. KALINOWSKI (Warszawa), BENIAMIN LENARCIK (Bydgoszcz), ZOFIA LIBUŚ (Gdańsk), JAN MAŁYSZKO (Kielce), BOGDAN MARCINIEC (Poznań), ZOFIA MICHALSKA (Łódź), ROMAN MIERZECKI (Warszawa), WŁADYSŁAW RUDZIŃSKI (Lublin), ZOFIA STASICKA (Kraków), JAN SZYMANOWSKI (Poznań), JÓZEF ŚLIWIOK (Katowice) KOMITET REDAKCYJNY BOGDAN BURCZYK, JERZY P. HAWRANEK, ADAM JEZIERSKI, ADOLF KISZĄ. LUDWIK KOMOROWSKI, ZDZISŁAW LATAJKA, PRZEMYSŁAW MASTALERZ, IGNACY Z. SIEMION, MIROSŁAW SOROKA, MARIA SUSZYŃSKA REDAKTOR NACZELNY JÓZEF J. ZIOŁKOWSKI SEKRETARZ REDAKCJI KRYSTYNA MARKSOWA Korespondencję należy kierować pod adresem: Redakcja „Wiadomości Chemicznych” ul. F. Joliot-Curie 14, 50-383 Wrocław teł. 375 73 89, tel./fax 322 14 06 INTERNET (English abstracts) http://www.chem.uni.wroc.plAviadchem.htm © Copyright by Redakcja „Wiadomości Chemicznych”, Wrocław 2004 ISSN 0043-5104 Maszynopis niniejszego numeru przekazano Wydawcy w lipcu 2004 Przygotowanie do druku i druk: Firma Wydawnicza K-2, ul. Konopnickiej 6, 00-491 Warszawa, tel./fax: (22) 628-97-66 WIADOMOŚCI 2004,58,7-8 chem iczne PL ISSN 0043-5104 <. f •O’’’ SEMIFLUOROWANE ALKANY (SFA) - WŁAŚCIWOŚCI W CIELE STAŁYM, ROZTWORACH I NA GRANICACH MIĘDZYFAZOWYCH SEMIFLUORINATED ALKANES (SFA)- PROPERTIES IN BULK SOLID, IN SOLUTIONS AND AT INTERFACES Marcin Broniatowski, Patrycja Dynarowicz-Łątka Zakład Chemii Ogólnej, Wydział Chemii Uniwersytetu Jagiellońskiego, ul. Ingardena 3, 30-060 Kraków Abstract Wstęp 1. Metodyka syntezy 2. Właściwości SFA w ciele stałym 2.1. -

Tonic Gabaergic Inhibition As a New Way to Regulate Mesolimbic Dopamine System
Tonic GABAergic inhibition as a new way to regulate mesolimbic dopamine system Elena Vashchinkina Institute of Biomedicine, Pharmacology University of Helsinki Academic Dissertation To be presented with the permission of the Medical Faculty of the University of Helsinki, for public examination in lecture hall 3, Biomedicum Helsinki 1, Haartmaninkatu 8, on December 13th 2013 at 12 noon Helsinki 2013 Supervisor Professor Esa R. Korpi, MD PhD Institute of Biomedicine, Pharmacology Faculty of Medicine University of Helsinki, Finland Reviewers Docent Mikko Airavaara, PhD Institute of Biotechnology University of Helsinki, Finland Docent Tarja Stenberg, MD PhD Institute of Biomedicine, Physiology University of Helsinki, Finland Dissertation Opponent Professor Kimmo Jensen, MD PhD Department of Biomedicine Aarhus University, Denmark ISBN 978-952-10-9636-5 (paperback) ISBN 978-952-10-9637-2 (PDF) http://ethesis.helsinki.fi Unigrafia OY Helsinki 2013 TABLE OF CONTENTS Abstract ......................................................................................................................... 1 Original publications ................................................................................................... 2 Abbreviations ............................................................................................................... 3 Glossary of terms ......................................................................................................... 4 1 INTRODUCTION .................................................................................................... -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0