"Amidohydrolase Superfamily"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded 10/5/2021 9:43:05 AM
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Aromatic side-chain flips orchestrate the conformational sampling of functional loops in Cite this: Chem. Sci.,2021,12,9318 † All publication charges for this article human histone deacetylase 8 have been paid for by the Royal Society a a bcd of Chemistry Vaibhav Kumar Shukla, ‡ Lucas Siemons, ‡ Francesco L. Gervasio and D. Flemming Hansen *a Human histone deacetylase 8 (HDAC8) is a key hydrolase in gene regulation and an important drug-target. High-resolution structures of HDAC8 in complex with substrates or inhibitors are available, which have provided insights into the bound state of HDAC8 and its function. Here, using long all-atom unbiased molecular dynamics simulations and Markov state modelling, we show a strong correlation between the conformation of aromatic side chains near the active site and opening and closing of the surrounding functional loops of HDAC8. We also investigated two mutants known to allosterically downregulate the enzymatic activity of HDAC8. Based on experimental data, we hypothesise that I19S-HDAC8 is unable to Received 6th April 2021 Creative Commons Attribution 3.0 Unported Licence. release the product, whereas both product release and substrate binding are impaired in the S39E- Accepted 27th May 2021 HDAC8 mutant. The presented results deliver detailed insights into the functional dynamics of HDAC8 DOI: 10.1039/d1sc01929e and provide a mechanism for the substantial downregulation caused by allosteric mutations, including rsc.li/chemical-science a disease causing one. Introduction II (HDAC-4, -5, -6, -7, -9, and HDAC10), and class III (SIRT1-7) have sequence similarity to yeast Rpd3, Hda1, and Sir2, Acetylation of lysine side chains occurs as a co-translation or respectively, whereas class IV (HDAC11) shares sequence simi- 2 This article is licensed under a post-translational modication of proteins and was rst iden- larity with both class I and II proteins. -

Atrazine Chlorohydrolase from Pseudomonas Sp
JOURNAL OF BACTERIOLOGY, Aug. 1996, p. 4894–4900 Vol. 178, No. 16 0021-9193/96/$04.0010 Copyright q 1996, American Society for Microbiology Atrazine Chlorohydrolase from Pseudomonas sp. Strain ADP: Gene Sequence, Enzyme Purification, and Protein Characterization† 1,2 2,3,4 1,2,4 MERVYN L. DE SOUZA, MICHAEL J. SADOWSKY, AND LAWRENCE P. WACKETT * Department of Biochemistry and Biological Processes Technology Institute,1 Department of Soil, Water, and Climate,3 Department of Microbiology,4 and Center for Biodegradation Research and Informatics,2 University of Minnesota, St. Paul, Minnesota 55108 Received 26 April 1996/Accepted 30 May 1996 Pseudomonas sp. strain ADP metabolizes atrazine to carbon dioxide and ammonia via the intermediate hydroxyatrazine. The genetic potential to produce hydroxyatrazine was previously attributed to a 1.9-kb AvaI DNA fragment from strain ADP (M. L. de Souza, L. P. Wackett, K. L. Boundy-Mills, R. T. Mandelbaum, and M. J. Sadowsky, Appl. Environ. Microbiol. 61:3373–3378, 1995). In this study, sequence analysis of the 1.9-kb AvaI fragment indicated that a single open reading frame, atzA, encoded an activity transforming atrazine to hydroxyatrazine. The open reading frame for the chlorohydrolase was determined by sequencing to be 1,419 nucleotides and encodes a 473-amino-acid protein with a predicted subunit molecular weight of 52,421. The deduced amino acid sequence matched the first 10 amino acids determined by protein microsequencing. The protein AtzA was purified to homogeneity by ammonium sulfate precipitation and anion-exchange chroma- tography. The subunit and holoenzyme molecular weights were 60,000 and 245,000 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration chromatography, respectively. -

Development of a Phage Display Library for Discovery of Antigenic Brucella Peptides Jeffrey Williams Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2018 Development of a phage display library for discovery of antigenic Brucella peptides Jeffrey Williams Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Microbiology Commons Recommended Citation Williams, Jeffrey, "Development of a phage display library for discovery of antigenic Brucella peptides" (2018). Graduate Theses and Dissertations. 16896. https://lib.dr.iastate.edu/etd/16896 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Development of a phage display library for discovery of antigenic Brucella peptides by Jeffrey Williams A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Microbiology Program of Study Committee: Bryan H. Bellaire, Major Professor Steven Olsen Steven Carlson The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University -
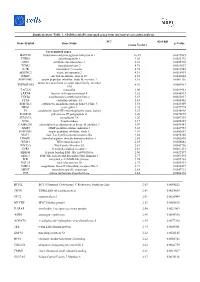
Supplementary Table 1. All Differentially Expressed Genes from Microarray Screening Analysis
Supplementary Table 1. All differentially expressed genes from microarray screening analysis. FCa (Gal-KD Gene Symbol Gene Name p-Value versus Vector) Up-regulated genes HAPLN1 hyaluronan and proteoglycan link protein 1 10.49 0.0027085 THBS1 thrombospondin 1 9.20 0.0022192 ODC1 ornithine decarboxylase 1 6.38 0.0055776 TGM2 transglutaminase 2 4.76 0.0015627 IL7R interleukin 7 receptor 4.75 0.0017245 SERINC2 serine incorporator 2 4.51 0.0014919 ITM2C integral membrane protein 2C 4.32 0.0044644 SERPINB7 serpin peptidase inhibitor, clade B, member 7 4.18 0.0081136 tumor necrosis factor receptor superfamily, member TNFRSF10D 4.01 0.0085561 10d TAGLN transgelin 3.90 0.0099963 LRRN4 leucine rich repeat neuronal 4 3.82 0.0046513 TGFB2 transforming growth factor beta 2 3.51 0.0035017 CPA4 carboxypeptidase A4 3.43 0.0008452 EPB41L3 erythrocyte membrane protein band 4.1-like 3 3.34 0.0025309 NRG1 neuregulin 1 3.28 0.0079724 F3 coagulation factor III (thromboplastin, tissue factor) 3.27 0.0038968 POLR3G polymerase III polypeptide G 3.26 0.0070675 SEMA7A semaphorin 7A 3.20 0.0087335 NT5E 5-nucleotidase 3.17 0.0036353 CAMK2N1 calmodulin-dependent protein kinase II inhibitor 1 3.07 0.0090141 TIMP3 TIMP metallopeptidase inhibitor 3 3.03 0.0047953 SERPINE1 serpin peptidase inhibitor, clade E 2.97 0.0053652 MALL mal, T-cell differentiation protein-like 2.88 0.0078205 DDAH1 dimethylarginine dimethylaminohydrolase 1 2.86 0.0002895 WDR3 WD repeat domain 3 2.85 0.0058842 WNT5A Wnt Family Member 5A 2.81 0.0043796 GPR1 G protein-coupled receptor 1 2.81 0.0021313 -

The Polycomb Group Genebmi1regulates Antioxidant
The Journal of Neuroscience, January 14, 2009 • 29(2):529–542 • 529 Neurobiology of Disease The Polycomb Group Gene Bmi1 Regulates Antioxidant Defenses in Neurons by Repressing p53 Pro-Oxidant Activity Wassim Chatoo,1* Mohamed Abdouh,1* Jocelyn David,1 Marie-Pier Champagne,1 Jose´ Ferreira,2 Francis Rodier,4 and Gilbert Bernier1,3 1Developmental Biology Laboratory and 2Department of Pathology, Maisonneuve-Rosemont Hospital, Montreal, Quebec, Canada H1T 2M4, 3Department of Ophthalmology, University of Montreal, Montreal, Quebec, Canada H3T 1J4, and 4Lawrence Berkeley National Laboratory, Berkeley, California 94720 Aging may be determined by a genetic program and/or by the accumulation rate of molecular damages. Reactive oxygen species (ROS) generated by the mitochondrial metabolism have been postulated to be the central source of molecular damages and imbalance between levels of intracellular ROS and antioxidant defenses is a characteristic of the aging brain. How aging modifies free radicals concentrations and increases the risk to develop most neurodegenerative diseases is poorly understood, however. Here we show that the Polycomb group and oncogene Bmi1 is required in neurons to suppress apoptosis and the induction of a premature aging-like program characterized by reduced antioxidant defenses. Before weaning, Bmi1 Ϫ/Ϫ mice display a progeroid-like ocular and brain phenotype, while Bmi1ϩ/ Ϫ mice, although apparently normal, have reduced lifespan. Bmi1 deficiency in neurons results in increased p19 Arf/p53 levels, abnormally high ROS concentrations, and hypersensitivity to neurotoxic agents. Most Bmi1 functions on neurons’ oxidative metabolism are genetically linked to repression of p53 pro-oxidant activity, which also operates in physiological conditions. In Bmi1 Ϫ/Ϫ neurons, p53 and corepres- sors accumulate at antioxidant gene promoters, correlating with a repressed chromatin state and antioxidant gene downregulation. -

Characterization of the Scavenger Cell Proteome in Mouse and Rat Liver
Biol. Chem. 2021; 402(9): 1073–1085 Martha Paluschinski, Cheng Jun Jin, Natalia Qvartskhava, Boris Görg, Marianne Wammers, Judith Lang, Karl Lang, Gereon Poschmann, Kai Stühler and Dieter Häussinger* Characterization of the scavenger cell proteome in mouse and rat liver + https://doi.org/10.1515/hsz-2021-0123 The data suggest that the population of perivenous GS Received January 25, 2021; accepted July 4, 2021; scavenger cells is heterogeneous and not uniform as previ- published online July 30, 2021 ously suggested which may reflect a functional heterogeneity, possibly relevant for liver regeneration. Abstract: The structural-functional organization of ammonia and glutamine metabolism in the liver acinus involves highly Keywords: glutaminase; glutamine synthetase; liver specialized hepatocyte subpopulations like glutamine syn- zonation; proteomics; scavenger cells. thetase (GS) expressing perivenous hepatocytes (scavenger cells). However, this cell population has not yet been char- acterized extensively regarding expression of other genes and Introduction potential subpopulations. This was investigated in the present study by proteome profiling of periportal GS-negative and There is a sophisticated structural-functional organization in perivenous GS-expressing hepatocytes from mouse and rat. the liver acinus with regard to ammonium and glutamine Apart from established markers of GS+ hepatocytes such as metabolism (Frieg et al. 2021; Gebhardt and Mecke 1983; glutamate/aspartate transporter II (GLT1) or ammonium Häussinger 1983, 1990). Periportal hepatocytes express en- transporter Rh type B (RhBG), we identified novel scavenger zymes required for urea synthesis such as the rate-controlling cell-specific proteins like basal transcription factor 3 (BTF3) enzyme carbamoylphosphate synthetase 1 (CPS1) and liver- and heat-shock protein 25 (HSP25). -

Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_001591825Cyc: Bacillus vietnamensis NBRC 101237 Cellular Overview Connections between pathways are omitted for legibility. Anamika Kothari sn-glycerol phosphate phosphate pro phosphate phosphate phosphate thiamine molybdate D-xylose D-ribose glutathione 3-phosphate D-mannitol L-cystine L-djenkolate lanthionine α,β-trehalose phosphate phosphate [+ 3 more] α,α-trehalose predicted predicted ABC ABC FliY ThiT XylF RbsB RS10935 UgpC TreP PutP RS10200 PstB PstB RS10385 RS03335 RS20030 RS19075 transporter transporter of molybdate of phosphate α,β-trehalose 6-phosphate L-cystine D-xylose D-ribose sn-glycerol D-mannitol phosphate phosphate thiamine glutathione α α phosphate phosphate phosphate phosphate L-djenkolate 3-phosphate , -trehalose 6-phosphate pro 1-phosphate lanthionine molybdate phosphate [+ 3 more] Metabolic Regulator Amino Acid Degradation Amine and Polyamine Biosynthesis Macromolecule Modification tRNA-uridine 2-thiolation Degradation ATP biosynthesis a mature peptidoglycan a nascent β an N-terminal- -

5'-Nucleotidases, Nucleosides and Their Distribution in the Brain: Patho- Logical and Therapeutic Implications
Send Orders for Reprints to [email protected] Current Medicinal Chemistry, 2013, 20, 4217-4240 4217 5'-Nucleotidases, Nucleosides and their Distribution in the Brain: Patho- logical and Therapeutic Implications Zsolt Kovács1,*, Árpád Dobolyi2, Katalin A. Kékesi3,4 and Gábor Juhász3 1Department of Zoology, University of West Hungary, Savaria Campus, Szombathely, Hungary; 2Laboratory of Neuro- morphology, Department of Anatomy, Histology and Embryology, Semmelweis University and the Hungarian Academy of Sciences, Budapest, Hungary; 3Laboratory of Proteomics, Institute of Biology, Eötvös Loránd University, Budapest, Hungary; 4 Department of Physiology and Neurobiology, Eötvös Loránd University, Budapest, Hungary Abstract: Elements of the nucleoside system (nucleoside levels, 5’-nucleotidases (5’NTs) and other nucleoside metabolic enzymes, nucleoside transporters and nucleoside receptors) are unevenly distributed in the brain, suggesting that nucleo- sides have region-specific functions in the human brain. Indeed, adenosine (Ado) and non-Ado nucleosides, such as guanosine (Guo), inosine (Ino) and uridine (Urd), modulate both physiological and pathophysiological processes in the brain, such as sleep, pain, memory, depression, schizophrenia, epilepsy, Huntington’s disease, Alzheimer’s disease and Parkinson’s disease. Interactions have been demonstrated in the nucleoside system between nucleoside levels and the ac- tivities of nucleoside metabolic enzymes, nucleoside transporters and Ado receptors in the human brain. Alterations in the nucleoside system may induce pathological changes, resulting in central nervous system (CNS) diseases. Moreover, sev- eral CNS diseases such as epilepsy may be treated by modulation of the nucleoside system, which is best achieved by modulating 5’NTs, as 5’NTs exhibit numerous functions in the CNS, including intracellular and extracellular formation of nucleosides, termination of nucleoside triphosphate signaling, cell adhesion, synaptogenesis and cell proliferation. -

Biochemical Characterization and Comparison of Aspartylglucosaminidases Secreted in Venom of the Parasitoid Wasps Asobara Tabida and Leptopilina Heterotoma
RESEARCH ARTICLE Biochemical characterization and comparison of aspartylglucosaminidases secreted in venom of the parasitoid wasps Asobara tabida and Leptopilina heterotoma Quentin Coulette1, SeÂverine Lemauf2, Dominique Colinet2, Geneviève PreÂvost1, Caroline Anselme1, Marylène Poirie 2, Jean-Luc Gatti2* a1111111111 1 Unite ªEcologie et Dynamique des Systèmes AnthropiseÂsº (EDYSAN, FRE 3498 CNRS-UPJV), Universite de Picardie Jules Verne, Amiens, France, 2 Universite CoÃte d'Azur, INRA, CNRS, ISA, Sophia Antipolis, a1111111111 France a1111111111 a1111111111 * [email protected] a1111111111 Abstract Aspartylglucosaminidase (AGA) is a low-abundance intracellular enzyme that plays a key OPEN ACCESS role in the last stage of glycoproteins degradation, and whose deficiency leads to human Citation: Coulette Q, Lemauf S, Colinet D, PreÂvost aspartylglucosaminuria, a lysosomal storage disease. Surprisingly, high amounts of AGA- G, Anselme C, Poirie M, et al. (2017) Biochemical characterization and comparison of like proteins are secreted in the venom of two phylogenetically distant hymenopteran para- aspartylglucosaminidases secreted in venom of the sitoid wasp species, Asobara tabida (Braconidae) and Leptopilina heterotoma (Cynipidae). parasitoid wasps Asobara tabida and Leptopilina These venom AGAs have a similar domain organization as mammalian AGAs. They share heterotoma. PLoS ONE 12(7): e0181940. https:// with them key residues for autocatalysis and activity, and the mature - and -subunits also doi.org/10.1371/journal.pone.0181940 α β form an (αβ)2 structure in solution. Interestingly, only one of these AGAs subunits (α for Editor: Erjun Ling, Institute of Plant Physiology and AtAGA and for LhAGA) is glycosylated instead of the two subunits for lysosomal human Ecology Shanghai Institutes for Biological β Sciences, CHINA AGA (hAGA), and these glycosylations are partially resistant to PGNase F treatment. -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Biosynthesis of Natural Products Containing Β-Amino Acids
Natural Product Reports Biosynthesis of natural products containing β -amino acids Journal: Natural Product Reports Manuscript ID: NP-REV-01-2014-000007.R1 Article Type: Review Article Date Submitted by the Author: 21-Apr-2014 Complete List of Authors: Kudo, Fumitaka; Tokyo Institute Of Technology, Department of Chemistry Miyanaga, Akimasa; Tokyo Institute Of Technology, Department of Chemistry Eguchi, T; Tokyo Institute Of Technology, Department of Chemistry and Materials Science Page 1 of 20 Natural Product Reports NPR RSC Publishing REVIEW Biosynthesis of natural products containing βββ- amino acids Cite this: DOI: 10.1039/x0xx00000x Fumitaka Kudo, a Akimasa Miyanaga, a and Tadashi Eguchi *b Received 00th January 2014, We focus here on β-amino acids as components of complex natural products because the presence of β-amino acids Accepted 00th January 2014 produces structural diversity in natural products and provides characteristic architectures beyond that of ordinary DOI: 10.1039/x0xx00000x α-L-amino acids, thus generating significant and unique biological functions in nature. In this review, we first survey the known bioactive β-amino acid-containing natural products including nonribosomal peptides, www.rsc.org/ macrolactam polyketides, and nucleoside-β-amino acid hybrids. Next, the biosynthetic enzymes that form β-amino acids from α-amino acids and de novo synthesis of β-amino acids are summarized. Then, the mechanisms of β- amino acid incorporation into natural products are reviewed. Because it is anticipated that the rational swapping of the β-amino acid moieties with various side chains and stereochemistries by biosynthetic engineering should lead to the creation of novel architectures and bioactive compounds, the accumulation of knowledge regarding β- amino acid-containing natural product biosynthetic machinery could have a significant impact in this field. -

Biodegradation of Atrazine by Atrazine Chlorohydrolase: Characterization of Mutant Enzyme and Immobilization System for Water Purification
Biodegradation of Atrazine by Atrazine Chlorohydrolase: Characterization of Mutant Enzyme and Immobilization System for Water Purification. A THESIS SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Amit Aggarwal IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE Lawrence P. Wackett & Ping Wang JANUARY 2012 © Amit Aggarwal 2011 Acknowledgement I want to thank my advisor, Dr. Larry Wackett, for the opportunity to work in his laboratory, his support and patience, and for allowing me to work independently and always being there to guide me. I would like to thank my mentor Dr. Jennifer Seffernick, for training me on different techniques in laboratory, and patiently helping me out throughout my association with the lab and teaching me good laboratory practices for research. Thank you so much for taking me under your wing. You are the best mentor I ever had. I would also like to thank Dr. Alptekin Aksan and Dr. Mike Sadowsky for guiding us throughout the immobilization of atrazine chlorohydrolase project. Dr. Alptekin Aksan guided us in development of a robust and efficient immobilization system and Dr. Mike Sadowsky provided us with valuable direction about making the immobilization system safe to be used in water purifications systems. I would like to thank my administrative adviser Dr. Ping Wang for always being supportive of my work and helping me out with planning my degree and ensuring that I meet all requirements in time. I am thankful to each of my committee members, Dr. Larry Wackett, Dr. Ping Wang and Dr. Jonathan Schilling for their time, for reading my thesis and for all the helpful suggestions.