Large B Cell Diffuse Lymphoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Cutaneous Anaplastic Large Cell Lymphoma / Lymphomatoid
Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders Definition A spectrum of related conditions originating from transformed or activated CD30-positive T-lymphocytes May coexist in individual patients Clonally related Overlapping clinical and/or histological features Clinical, histologic, and phenotypic characteristics required for diagnosis Types 1. Primary cutaneous anaplastic large cell lymphoma (C-ALCL) 2. Lymphomatoid papulosis 3. Borderline lesions C-ALCL: Definition T-cell lymphoma, presenting in the skin and consisting of anaplastic lymphoid cells, the majority of which are CD30- positive Distinction from: (a) systemic ALCL with cutaneous involvement, and (b) secondary high-grade lymphomas with CD30 expression In nearly all patients disease is limited to the skin at the time of diagnosis Assessed by meticulous staging Patients should not have other subtypes of lymphoma C-ALCL: Synonyms Lukes-Collins: Not listed (T-immunoblastic) Kiel: Anaplastic large cell Working Formulation: Various categories (diffuse large cell; immunoblastic) REAL: Primary cutaneous anaplastic large cell (CD30+) lymphoma Related terms: Regressing atypical histiocytosis; Ki-1 lymphoma C-ALCL: Epidemiology 25% of the T-cell lymphomas arising primarily in the skin. Predominantly in adults/elderly and rare in children. The male to female ratio is 1.5-2.0:1. C-ALCL: Sites of Involvement The disease is nearly always limited to the skin at the time of diagnosis Extracutaneous dissemination may occur Mainly regional lymph nodes Involvement of other organs is rare C-ALCL: Clinical Features Most present solitary or localized skin lesions which may be tumors, nodules or (more rarely) papules Multicentric cutaneous disease occurs in 20% Lesions may show partial or complete spontaneous regression (similar to lymphomatoid papulosis) Cutaneous relapses are frequent Extracutaneous dissemination occurs in approximately 10% of the patients. -

Hematopoietic and Lymphoid Neoplasm Coding Manual
Hematopoietic and Lymphoid Neoplasm Coding Manual Effective with Cases Diagnosed 1/1/2010 and Forward Published August 2021 Editors: Jennifer Ruhl, MSHCA, RHIT, CCS, CTR, NCI SEER Margaret (Peggy) Adamo, BS, AAS, RHIT, CTR, NCI SEER Lois Dickie, CTR, NCI SEER Serban Negoita, MD, PhD, CTR, NCI SEER Suggested citation: Ruhl J, Adamo M, Dickie L., Negoita, S. (August 2021). Hematopoietic and Lymphoid Neoplasm Coding Manual. National Cancer Institute, Bethesda, MD, 2021. Hematopoietic and Lymphoid Neoplasm Coding Manual 1 In Appreciation NCI SEER gratefully acknowledges the dedicated work of Drs, Charles Platz and Graca Dores since the inception of the Hematopoietic project. They continue to provide support. We deeply appreciate their willingness to serve as advisors for the rules within this manual. The quality of this Hematopoietic project is directly related to their commitment. NCI SEER would also like to acknowledge the following individuals who provided input on the manual and/or the database. Their contributions are greatly appreciated. • Carolyn Callaghan, CTR (SEER Seattle Registry) • Tiffany Janes, CTR (SEER Seattle Registry) We would also like to give a special thanks to the following individuals at Information Management Services, Inc. (IMS) who provide us with document support and web development. • Suzanne Adams, BS, CTR • Ginger Carter, BA • Sean Brennan, BS • Paul Stephenson, BS • Jacob Tomlinson, BS Hematopoietic and Lymphoid Neoplasm Coding Manual 2 Dedication The Hematopoietic and Lymphoid Neoplasm Coding Manual (Heme manual) and the companion Hematopoietic and Lymphoid Neoplasm Database (Heme DB) are dedicated to the hard-working cancer registrars across the world who meticulously identify, abstract, and code cancer data. -

Indolent Non-Hodgkin's Lymphomas
Follicular and Low-Grade Non-Hodgkin Lymphomas (Indolent Lymphomas) Stefan K Barta, M.D., M.S. Associate Professor of Medicine Leader, T Cell Lymphoma Program Perelman Center for Advanced Medicine Facts and Figures: Non-Hodgkin Lymphomas • Most common blood cancer • 7th most common cancer in the US3 • 71,850 new cases in the US in 20151 • 19,790 died of NHL in 20151 • About 549,625 people are living with a history of NHL (2012)1 • 85% of all NHLs are B-cell lymphomas2 • Follicular lymphoma = 2nd most common type, ~25% of all NHLs4 1 http://seer.cancer.gov/statfacts/html/nhl.html. 2 ACS. Detailed Guide (revised January 21, 2000): Non-Hodgkin’s Lymphoma. 3 http://www.cancer.gov/cancertopics/types/commoncancers 4 Blood 89: 3909, 1997 The Immune System T- CELLS B- CELLS Cellular immunity: Humoral immunity: helper + cytotoxic T-cells antibodies Lymphatic System Lymph Node Anatomy Lymph Node: Microscopic View germinal center Lymphocyte: Microscopic View Causes Possible cause(s): • chemical exposures (pesticides, fertilizers or solvents) • individuals with compromised immune systems • heredity • infections (e.g. H. pylori, Hep C, chlamydia trachomatis) • most patients have no clear risk factors • IN MOST CASES, THE EXACT CAUSE IS UNKNOWN Cellular Origins of Lymphomas & Leukemias PLEURIPOTENT STEM CELL ACUTE LEUKEMIAS LYMPHOID STEM CELL ACUTE LYMPHOBLASTIC LEUKEMIAS PRECURSOR T - CELL PRECURSOR B - CELL LYMPHOBLASTIC LYMPHOMAS / LEUKEMIAS MATURE T - CELL MATURE B - CELL NON-HODGKIN LYMPHOMAS / CHRONIC LYMPHOCYTIC LEUKEMIA LYMPH NODES, EXTRANODAL -
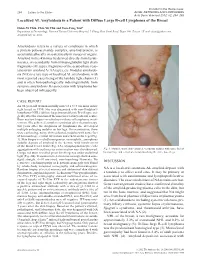
Localized AL Amyloidosis in a Patient with Diffuse Large B-Cell Lymphoma of the Breast
Included in the theme issue: 284 Letters to the Editor ACNE, RETINOIDS AND LYMPHOMAS Acta Derm Venereol 2012; 92: 284–285 Localized AL Amyloidosis in a Patient with Diffuse Large B-cell Lymphoma of the Breast Hsien-Yi Chiu, Chia-Yu Chu and Tsen-Fang Tsai* Department of Dermatology, National Taiwan University Hospital, 7 Chung-Shan South Road, Taipei 100, Taiwan. *E-mail: [email protected] Accepted July 11, 2011. Amyloidosis refers to a variety of conditions in which a protein polysaccharide complex, amyloid protein, is accumulated locally or systemically in tissues or organs. Amyloid in the skin may be derived directly from kerati- nocytes, or secondarily from immunoglobulin light chain fragments (AL type), fragments of the acute-phase reac- tant serum amyloid A (AA type), etc. Nodular amyloido- sis (NA) is a rare type of localized AL amyloidosis, with most reported cases being of the lambda light chains (1) and is often histopathologically indistinguishable from systemic amyloidosis. Its association with lymphoma has been observed infrequently. CASE REPORT An 88-year-old woman initially noticed a 5×3 cm mass in her right breast in 1990. She was diagnosed with non-Hodgkin’s lymphoma (NHL) (diffuse large immunoblastic B-cell type, sta- ge IE) after the excision of the mass in a tertiary referral centre. Bone marrow biopsy revealed no evidence of lymphoma invol- vement. She achieved complete remission after chemotherapy. Six years after the diagnosis of lymphoma she developed multiple enlarging nodules on her legs. On examination, there were coalescing, waxy, skin-coloured, nodules with some foci of haemorrhage, central ulceration and crusts on the legs (Fig. -

REVIEW Anti-CD20-Based Therapy of B Cell Lymphoma: State of The
Leukemia (2002) 16, 2004–2015 2002 Nature Publishing Group All rights reserved 0887-6924/02 $25.00 www.nature.com/leu REVIEW Anti-CD20-based therapy of B cell lymphoma: state of the art C Kosmas1, K Stamatopoulos2, N Stavroyianni2, N Tsavaris3 and T Papadaki4 1Department of Medicine, 2nd Division of Medical Oncology, ‘Metaxa’ Cancer Hospital, Piraeus, Greece; 2Department of Hematology, G Papanicolaou General Hospital, Thessaloniki, Greece; 3Oncology Unit, Department of Pathophysiology, Athens University School of Medicine, Laikon General Hospital, Athens, Greece; and 4Hemopathology Department, Evangelismos Hospital, Athens, Greece Over the last 5 years, studies applying the chimeric anti-CD20 ficulties in identifying a completely tumor-specific target; (2) MAb have renewed enthusiasm and triggered world-wide appli- the impracticality of constructing a unique antibody for each cation of anti-CD20 MAb-based therapies in B cell non-Hodg- kin’s lymphoma (NHL). Native chimeric anti-CD20 and isotope- patient; (3) the development of an immune response to murine 6 labeled murine anti-CD20 MAbs are currently employed with immunoglobulins (human anti-mouse antibodies, HAMA). By encouraging results as monotherapy or in combination with the end of the 1980s enthusiasm for therapeutic MAbs was conventional chemotherapy and in consolidation of remission waning; murine native (unconjugated), radioactively labeled after treatments with curative intent (ie after/ in combination or toxin-conjugated MAbs failed to yield significant clinical with high-dose chemotherapy and hematopoietic stem cell responses; moreover, they were not uncommonly associated rescue). On the available experience, anti-CD20 MAb-based therapeutic strategies will be increasingly integrated in the with toxicities, predominantly in the form of serum sickness treatment of B cell NHL and related malignancies. -

Molecular and Immunological Dissection of Diffuse Large
Leukemia (1999) 13, 1441–1447 1999 Stockton Press All rights reserved 0887-6924/99 $15.00 http://www.stockton-press.co.uk/leu ,Molecular and immunological dissection of diffuse large B cell lymphoma: CD5؉ and CD5؊ with CD10؉ groups may constitute clinically relevant subtypes S Harada1,2, R Suzuki1, K Uehira3, Y Yatabe4, Y Kagami3, M Ogura3, H Suzuki5, A Oyama6, Y Kodera7, R Ueda2, Y Morishima3, S Nakamura4 and M Seto1 1Laboratory of Chemotherapy, Aichi Cancer Center Research Institute; 3Department of Hematology and Chemotherapy, 4Department of Pathology and Clinical Laboratories, Aichi Cancer Center Hospital; 2Second Department of Internal Medicine, Nagoya City University School of Medicine; 5Department of Internal Medicine, Okazaki Municipal Hospital; 6Department of Internal Medicine, Aichi Prefectural Hospital; 7Department of Internal Medicine, Japanese Red Cross, Nagoya First Hospital, Japan Diffuse large B cell lymphoma (DLBL) constitutes the greatest genotypic parameters were found not to be helpful for deline- percentage of adult non-Hodgkin’s lymphomas and represents ating distinctive morphologic subgroups). DLBL accounts for a diverse spectrum of lymphoid neoplasms. Clinicopathologic, the greatest percentage (48%) of adult non-Hodgkin’s lym- phenotypic and genotypic findings were correlated and com- 4 pared for 63 DLBL cases to investigate whether they represent phoma (NHL) among Japanese patients and for about 40% clinically relevant subtypes. They were all cyclin D1 negative of initial NHL diagnoses in the United States.5 Among DLBL, and were phenotypically divided into three groups, ie group I only a few entities, eg primary mediastinal large B cell lym- + − + (CD5 type, n = 11), group II (CD5 CD10 type, n = 19), and phoma characterized by the fact that it has a peculiar disease − − = group III (CD5 CD10 type, n 33). -

V1 Madrid 3 Intro.Pdf
Clasificación de la OMS de las neoplasias linfoides is about to begin! Please silence your cellphones, beepers, person sitting next to you….. When Dr. Campo asked me to provide this introduction, I had to think long and hard before agreeing to prepare it for this course & deciding how to do it but….. he is a good friend so …… Clasificación de los linfomas. Evolución conceptual. Please do not be disappointed The remainder of this presentation is in American Style English However, this is an international story not from just one family tree nor one side of the Atlantic So let’s begin! S. Fairey Lymphoma classification over the last half- century: A tale of evolution & revolution The Rappaport classification 1966 1974 Lymphomas are neoplasms of the immune system and should be categorized as such! The “functional” lymphoma classifications Cells of the normal immune system Lukes & Collins, Cancer 34:1488, 1974 Morphology & immunophenotype R. D. Collins - Vanderbilt R. L. Lukes - USC 1974 K. Lennert Signing off on the Kiel classification The 1970’s were very exciting but tumultuous times – not everyone was on the same page! “It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness….” The 1970’s were very exciting but tumultuous times – not everyone was on the same page! “It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness….” Lancet 1974 7;2(7880): 586 Lancet 1974 7;2(7880): 586 NCI Working Formulation Cancer 49:2112, 1982 Morphologic classification based in large part on survival data from the late 1970s. -

An Overview of the Classification of Non-Hodgkin's Lymphomas: an Integration of Morphological and Phenotypical Concepts1
[CANCER RESEARCH (SUPPL.) 52, 5447s-5452s. October I. 1992| An Overview of the Classification of Non-Hodgkin's Lymphomas: An Integration of Morphological and Phenotypical Concepts1 Elaine S. Jaffe,2 Mark Raffeid, L. Jeffrey Medeiros, and Maryalice Stetler-Stevenson Hematopathology Section, Laboratory of Pathology, National Cancer Institute, NIH, Bethesda, Maryland 20982 Abstract As a result of this dissatisfaction, a variety of classification An analysis of trends in the incidence of non-Hodgkin's lymphoma schemes were proposed. Two of them, the Lukes and Collins Classification (3) and the Kiel Scheme (4), as proposed by Len- requires an understanding of individual disease entities within this broad group. The non-Hodgkin's lymphomas represent a diverse group nert and colleagues, are shown in Table 1. The complexity of of malignancies that have in common an origin from lymphoid cells. this terminology is obvious. All of these terms have been in Nevertheless, these disorders are heterogeneous in their clinical behav International Classification of Diseases for Oncology, and ior, morphological appearance, cellular origin, etiology, and pathogen- probably all are reflected in the SEER data at one point or esis. A modern classification of non-Hodgkin's lymphomas must include another. Moreover, if one tries to discern individual disease an integration of morphological, immunophenotypical, and molecular entities from these data, it is virtually impossible. concepts in order to delineate individual diseases within this broad The National Cancer Institute responded to this problem by group. Existing classification schemes such as the working formulation, funding a study to test the six major classification schemes while they may be useful in providing a guide to clinical management, cannot provide this information in the absence of other data. -

The Spectrum of B Cell Neoplasia
Henry Ford Hospital Medical Journal Volume 32 Number 1 Article 6 3-1984 The Spectrum of B Cell Neoplasia Michael J. Deegan Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Deegan, Michael J. (1984) "The Spectrum of B Cell Neoplasia," Henry Ford Hospital Medical Journal : Vol. 32 : No. 1 , 20-30. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol32/iss1/6 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp Med Vol 32, No 1, 1984 The Spectrum of B Cell Neoplasia Michael J. Deegan, MD^ Recenf progress in our understanding of the immune peutic implications of these discoveries are now being system and the development of new techniques that explored. This paper presents a concise overview of the permit the precise identification of lymphocytes have differentiation of human B lymphocytes, the surface and permitted a reexamination of lymphoid neoplasms. cytoplasmic markers that permit their recognition, and Most ofthe non-Hodgkin's lymphomas and lymphocytic the diverse tumors that are now known to be malignant leukemias have been characterized as T or B cell neo counterparts of normal B cell elements. Particular em plasms and have been shown to possess features similar phasis is placed on the utility of surface and cytoplasmic to those expressed by normal lymphoid cells at different immunoglobulin as unique B cell markers and on the stages of maturation. -

Uva-DARE (Digital Academic Repository)
UvA-DARE (Digital Academic Repository) CD20 monoclonal antibody therapy for B-cell lymphona van der Kolk, L.E. Publication date 2001 Link to publication Citation for published version (APA): van der Kolk, L. E. (2001). CD20 monoclonal antibody therapy for B-cell lymphona. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:29 Sep 2021 CC h a p t e Introductionn and outline of the thesis Introductionn and outline of the thesis Introduction n Low-gradee non-Hodgkin's lymphoma (NHL) Non-Hodgkin'ss lymphomas are a heterogeneous group of malignancies of mature lymphoid cells.. Over 90% of the non-Hodgkin's lymphomas is of B-cell origin. The clinical course variess from indolent to highly aggressive. -

Staging and Classification of Lymphoma
Staging and Classification of Lymphoma Ping Lu, MD In 2004, new cases of non-Hodgkin’s lymphoma in the United States were estimated at 54,370, representing 4% of all cancers and resulting 4% of all cancer deaths, and new cases of Hodgkin’s lymphoma were estimated at 7,880. The appropriate staging and management of lymphomas greatly depend on an accurate pathological diagnosis and classification. The recently established Revised European–American Classification of Lymphoid Neoplasms (REAL) and the subsequently adopted and updated World Health Organization (WHO) classification include modern cytogenetic, molecular, and immu- nologic techniques and knowledge and reach an international consensus on the clas- sification of lymphomas. This classification scheme represents an advance in our understanding of lymphomas and serves as an operative guideline for studying and diagnosing lymphomas. Imaging techniques always have served as staging and moni- toring tools for the clinical management of lymphomas. The understanding and adoption of the current classification system is important in refining the role of imaging modal- ities in the management of specific lymphoma. To help one understand the current classification, this current review gives a brief history of lymphoma classifications and summaries the recent classification schemes, including new entities, clinical staging methods, and clinical prognostic criteria. Semin Nucl Med 35:160-164 © 2005 Elsevier Inc. All rights reserved. uring past decades, because of the lack of knowledge according to their natural history, their response to ther- Din the lymphoma biology, the diagnosis and classifi- apy, and their survival in the patients recruited in the cation of lymphoma were based solely on morphology. -

Diffuse Large B-Cell Non-Hodgkin Lymphomas
British Journal of Cancer (1999) 79(11/12), 1770–1776 © 1999 Cancer Research Campaign Article no. bjoc.1998.0282 Diffuse large B-cell non-Hodgkin lymphomas: the clinical relevance of histological subclassification JW Baars1, D de Jong2, EM Willemse3, L Gras4, O Dalesio4, P v. Heerde2, PC Huygens5, H v.d. Lelie6 and AEG Kr v.d. Borne1,6 1Division of Medical Oncology, Department of Hematology, 2Department of Pathology, 3Department of Scientific and Patient Administration, 4Department of Biometrics, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121,1066 CX Amsterdam, The Netherlands; 5Department of Hematology, Academic Hospital of the Free University, de Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; 6Division of Internal Medicine, Department of Hematology, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands Summary In the REAL classification the diffuse large B-cell non-Hodgkin lymphomas (NHL) are grouped together, because subclassifications are considered to lack both reproducibility and clinical significance. Others, however, claim that patients with an immunoblastic NHL have a worse prognosis than patients with other types of diffuse large B-cell NHL. Therefore, we investigated the prognostic and clinical significance of histological subclassification of diffuse large B-cell NHL in a uniformly treated series of patients. For this retrospective study, all patients diagnosed as having an immunoblastic (IB) B-cell NHL by the Lymphoma Review Panel of the Comprehensive Cancer Center Amsterdam (CCCA) between 1984 and 1994, and treated according to the guidelines of the CCCA, were analysed. Patients with a centroblastic polymorphic subtype (CB-Poly) or centroblastic (CB) NHL by the Lymphoma Review Panel who were treated in the Netherlands Cancer Institute during the same period according to CCCA guidelines were used as reference groups.