Indolent Non-Hodgkin's Lymphomas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Cutaneous Anaplastic Large Cell Lymphoma / Lymphomatoid
Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders Definition A spectrum of related conditions originating from transformed or activated CD30-positive T-lymphocytes May coexist in individual patients Clonally related Overlapping clinical and/or histological features Clinical, histologic, and phenotypic characteristics required for diagnosis Types 1. Primary cutaneous anaplastic large cell lymphoma (C-ALCL) 2. Lymphomatoid papulosis 3. Borderline lesions C-ALCL: Definition T-cell lymphoma, presenting in the skin and consisting of anaplastic lymphoid cells, the majority of which are CD30- positive Distinction from: (a) systemic ALCL with cutaneous involvement, and (b) secondary high-grade lymphomas with CD30 expression In nearly all patients disease is limited to the skin at the time of diagnosis Assessed by meticulous staging Patients should not have other subtypes of lymphoma C-ALCL: Synonyms Lukes-Collins: Not listed (T-immunoblastic) Kiel: Anaplastic large cell Working Formulation: Various categories (diffuse large cell; immunoblastic) REAL: Primary cutaneous anaplastic large cell (CD30+) lymphoma Related terms: Regressing atypical histiocytosis; Ki-1 lymphoma C-ALCL: Epidemiology 25% of the T-cell lymphomas arising primarily in the skin. Predominantly in adults/elderly and rare in children. The male to female ratio is 1.5-2.0:1. C-ALCL: Sites of Involvement The disease is nearly always limited to the skin at the time of diagnosis Extracutaneous dissemination may occur Mainly regional lymph nodes Involvement of other organs is rare C-ALCL: Clinical Features Most present solitary or localized skin lesions which may be tumors, nodules or (more rarely) papules Multicentric cutaneous disease occurs in 20% Lesions may show partial or complete spontaneous regression (similar to lymphomatoid papulosis) Cutaneous relapses are frequent Extracutaneous dissemination occurs in approximately 10% of the patients. -

Clinical Utility of Recently Identified Diagnostic, Prognostic, And
Modern Pathology (2017) 30, 1338–1366 1338 © 2017 USCAP, Inc All rights reserved 0893-3952/17 $32.00 Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms Arantza Onaindia1, L Jeffrey Medeiros2 and Keyur P Patel2 1Instituto de Investigacion Marques de Valdecilla (IDIVAL)/Hospital Universitario Marques de Valdecilla, Santander, Spain and 2Department of Hematopathology, MD Anderson Cancer Center, Houston, TX, USA Genomic profiling studies have provided new insights into the pathogenesis of mature B-cell neoplasms and have identified markers with prognostic impact. Recurrent mutations in tumor-suppressor genes (TP53, BIRC3, ATM), and common signaling pathways, such as the B-cell receptor (CD79A, CD79B, CARD11, TCF3, ID3), Toll- like receptor (MYD88), NOTCH (NOTCH1/2), nuclear factor-κB, and mitogen activated kinase signaling, have been identified in B-cell neoplasms. Chronic lymphocytic leukemia/small lymphocytic lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, Burkitt lymphoma, Waldenström macroglobulinemia, hairy cell leukemia, and marginal zone lymphomas of splenic, nodal, and extranodal types represent examples of B-cell neoplasms in which novel molecular biomarkers have been discovered in recent years. In addition, ongoing retrospective correlative and prospective outcome studies have resulted in an enhanced understanding of the clinical utility of novel biomarkers. This progress is reflected in the 2016 update of the World Health Organization classification of lymphoid neoplasms, which lists as many as 41 mature B-cell neoplasms (including provisional categories). Consequently, molecular genetic studies are increasingly being applied for the clinical workup of many of these neoplasms. In this review, we focus on the diagnostic, prognostic, and/or therapeutic utility of molecular biomarkers in mature B-cell neoplasms. -

Hematopoietic and Lymphoid Neoplasm Coding Manual
Hematopoietic and Lymphoid Neoplasm Coding Manual Effective with Cases Diagnosed 1/1/2010 and Forward Published August 2021 Editors: Jennifer Ruhl, MSHCA, RHIT, CCS, CTR, NCI SEER Margaret (Peggy) Adamo, BS, AAS, RHIT, CTR, NCI SEER Lois Dickie, CTR, NCI SEER Serban Negoita, MD, PhD, CTR, NCI SEER Suggested citation: Ruhl J, Adamo M, Dickie L., Negoita, S. (August 2021). Hematopoietic and Lymphoid Neoplasm Coding Manual. National Cancer Institute, Bethesda, MD, 2021. Hematopoietic and Lymphoid Neoplasm Coding Manual 1 In Appreciation NCI SEER gratefully acknowledges the dedicated work of Drs, Charles Platz and Graca Dores since the inception of the Hematopoietic project. They continue to provide support. We deeply appreciate their willingness to serve as advisors for the rules within this manual. The quality of this Hematopoietic project is directly related to their commitment. NCI SEER would also like to acknowledge the following individuals who provided input on the manual and/or the database. Their contributions are greatly appreciated. • Carolyn Callaghan, CTR (SEER Seattle Registry) • Tiffany Janes, CTR (SEER Seattle Registry) We would also like to give a special thanks to the following individuals at Information Management Services, Inc. (IMS) who provide us with document support and web development. • Suzanne Adams, BS, CTR • Ginger Carter, BA • Sean Brennan, BS • Paul Stephenson, BS • Jacob Tomlinson, BS Hematopoietic and Lymphoid Neoplasm Coding Manual 2 Dedication The Hematopoietic and Lymphoid Neoplasm Coding Manual (Heme manual) and the companion Hematopoietic and Lymphoid Neoplasm Database (Heme DB) are dedicated to the hard-working cancer registrars across the world who meticulously identify, abstract, and code cancer data. -

Implications of Plasma Lymphoblastic Cells In
REVIEW ARTICLE Implications of Plasma Lymphoblastic Cells in Lymphoreticular Disorders: An Overview Marin Abraham1 , SV Sowmya2 , Roopa S Rao3,DominicAugustine4 , Vanishri C Haragannavar5 ABSTRACT Aim: The aim of this review was to emphasize the diverse morphologic features of plasma lymphoblastic cells in lymphoreticular disorders to arrive at a precise diagnosis. Background: The lymphoreticular system comprises of a group of cells with a common lineage and primary function of immunoregulation. Specific immunity is achieved by the combined effects of macrophages and lymphocytes, and, therefore, it is the lymphoreticular system. These cells are scattered in different parts of the body and share some functional characteristics. At both functional and anatomical levels, lymphoreticular tissue can be categorized into primary and secondary lymphoid organs that predominantly produce lymphocytes and plasma cells. Review results: The plasma lymphoblastic lesions/malignancies comprise of characteristic cells like buttock cells, cells with irregular nuclei, cells with cleaved nuclear outlines, etc. Identification of such cells amidst sheets of malignant lymphoblastic cells is challenging. However, sound knowledge about the morphology of these cells and their immunohistochemical panel of markers may provide a clue for diagnosis. Conclusion: The predominant cell types noted in plasma lymphoblastic lesions histopathologically are immature lymphocytes and plasma cells in their varied cell activity suggest the biologic behavior of the lesion. Clinical significance: Understanding and identifying the normal and pathological cellular and nuclear morphology of the lymphoreticular cells can aid in the definitive diagnosis of the plasma lymphoblastic disorders and predict its biological nature. Keywords: Hematopoietic stem cells, Immunoglobulins, Lymphoreticular system, Lymphoblasts, Plasmablasts. World Journal of Dentistry (2019): 10.5005/jp-journals-10015-1636 INTRODUCTION 1–5 Department of Oral Pathology and Microbiology, M. -

©Ferrata Storti Foundation
Lymphoproliferative Disorders original paper haematologica 2001; 86:1046-1050 Efficacy of anti-CD20 monoclonal http://www.haematologica.it/2001_10/1046.htm antibodies (Mabthera) in patients with progressed hairy cell leukemia FRANCESCO LAURIA, MARIAPIA LENOCI, LUCIANA ANNINO,* DONATELLA RASPADORI, GIUSEPPE MAROTTA, MONICA BOCCHIA, FRANCESCO FORCONI, SARA GENTILI, MICHELA LA MANDA,* SILVIA MARCONCINI, MONICA TOZZI, LUCA BALDINI,# PIER LUIGI ZINZANI,° ROBIN FOÀ* Department of Hematology, University of Siena; *Department of Hematology, University “La Sapienza”, Rome; °Institute of Correspondence: Francesco Lauria, MD, Department of Hematology “A. Sclavo” Hospital, via Tufi 1, 53100 Siena, Italy. Hematology and Clinical Oncology “Seragnoli”, University of Phone: international +39.0577.586798. Bologna; #Centro G. Marcora, University of Milan, Italy Fax: international +39.0577.586185. E-mail: [email protected] Background and Objectives. Recently, a chimeric Interpretation and Conclusions. On the basis of monoclonal antibody (MoAb) directed against the these preliminary results observed in 10 patients CD20 antigen (rituximab) has been successfully with progressed HCL, it appears that treatment with introduced in the treatment of several CD20-posi- anti-CD20 MoAb is safe and effective in at least tive B-cell neoplasias and particularly of follicular 50% of patients, particularly in those with a less lymphomas. Based on these premises we evaluat- evident bone marrow infiltration (≤ 50%) and in ed the efficacy and the toxicity of chimeric those previously -

Hairy Cell Leukemia: the Good News of a Bad Disease
CASO CLÍNICO Hairy Cell Leukemia: the good news of a bad disease Mónica Seidi, Guadalupe Benites, Almerindo Rego Hospital de Santo Espírito da Ilha Terceira Abstract Hairy Cell Leukemia (HCL) is an uncommon chronic B cell Lymphoproliferative disorder characterized by the accumulation of a small mature B cell lym- phoid cells with abundant cytoplasm and “hairy” projections within the peripheral blood smear, bone marrow and splenic red pulp. Most patients with HCL present with symptons related to splenomegaly or cytopenias, including some constitucional symptons, however one quarter of them is asymptomatic and is referred due to incidental findings. The authors decided to report a clinical case of hairy cells leukemia in an asymptomatic patient due to the rarity of this neoplasia (2% of all leukemias Galicia Clínica | Sociedade Galega de Medicina Interna and less than 1% of limphoids neoplasms) and because it corresponds to the most successfully treatable leukemia. Palabras clave: Citopenias. Esplenomegalia. Enfermedad linfoproliferativa. Leucemia de células peludas. Keywords: Cytopenias. Splenomegaly. Lymphoproliferative disease. Hairy cells leukemia Introduction clonal gamma peak. The CT abdominal scan revealed homogenea Hairy cell leukemia (HCL) is an uncommon B-cell lymphopro- splenomegaly with no limphadenopathy present. liferative disorder that affects adults, and was first reported We decided to admit the patient in the ward to perform invasive exams such as a bone marrow aspiration which showed 66% of as a distinct disease in 1958 -

Hairy Cell Leukemia
Hairy Cell Leukemia No. 16 in a series providing the latest information for patients, caregivers and healthcare professionals Introduction Highlights Hairy cell leukemia (HCL) is a rare, slow-growing y Hairy cell leukemia (HCL) is a rare, leukemia that starts in a B cell (B lymphocyte). B cells are slow-growing leukemia that starts in a B cell white blood cells that help the body fight infection and (also called B lymphocyte), a type of white are an important part of the body’s immune system. blood cell. Changes (mutations) in the genes of a B cell can cause it y Changes (mutations) in the genes of a to develop into a leukemia cell. Normally, a healthy B cell B cell can cause it to develop into a leukemia would stop dividing and eventually die. In HCL, genetic cell. In HCL, leukemic B cells are overproduced errors tell the B cell to keep growing and dividing. Every and infiltrate the bone marrow and spleen. cell that arises from the initial leukemia cell also has the They may also be found in the liver and lymph mutated DNA. As a result, the leukemia cells multiply nodes. These excess B cells are abnormal uncontrollably. They usually go on to infiltrate the bone and have projections that look like hairs marrow and spleen, and they may also invade the liver under a microscope. and lymph nodes. The disease is called “hairy cell” y Signs and symptoms of HCL include an leukemia because the leukemic cells have short, thin enlarged spleen and a decrease in normal projections on their surfaces that look like hairs when blood cell counts. -

Cancer Association of South Africa (CANSA) Fact Sheet on Adult Hairy Cell Leukaemia
Cancer Association of South Africa (CANSA) Fact Sheet on Adult Hairy Cell Leukaemia Introduction Leukaemia is a cancer of the blood forming system. Most types of leukaemia cause the bone marrow to make abnormal white blood cells. These abnormal cells can get into the bloodstream and circulate around the body. [Picture Credit: Hairy Cell Leukaemia] Adult Hairy Cell Leukaemia (HCL) Hairy cell leukaemia is a rare, slow-growing cancer of the blood in which the bone marrow makes too many B cells (lymphocytes), a type of white blood cell that fights infection. These excess B cells are abnormal and look "hairy" under a microscope. As the number of leukaemia cells increases, fewer healthy white blood cells, red blood cells and platelets are produced. This disease affects more men than women, and it occurs most commonly in middle-aged or older adults. It is considered a chronic disease because it may never completely disappear, although treatment can lead to remission for years. Joshi, A., Dhanushkodi, M., Ganesan, P., Radhakrishnan, V., Kannan, K., Mehra, N., Kalaiyarasi, J.P., Krupashankar, S., Sundersingh, S., Ganesan, T.S. & Sagar, T.G. 2020. “HCL is an uncommon B cell lympho-proliferative disorder with high remission rates. There is paucity of data on the long-term outcome of HCL from India. We retrospectively collected data from individual case records of patients with HCL who were treated in Cancer Institute, Chennai from January 2001 until January 2018. Sixteen patients were diagnosed with HCL and were treated with cladribine (81%), interferon (13%) and one patient received only best supportive care (6%). -
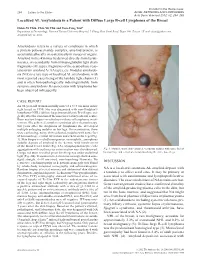
Localized AL Amyloidosis in a Patient with Diffuse Large B-Cell Lymphoma of the Breast
Included in the theme issue: 284 Letters to the Editor ACNE, RETINOIDS AND LYMPHOMAS Acta Derm Venereol 2012; 92: 284–285 Localized AL Amyloidosis in a Patient with Diffuse Large B-cell Lymphoma of the Breast Hsien-Yi Chiu, Chia-Yu Chu and Tsen-Fang Tsai* Department of Dermatology, National Taiwan University Hospital, 7 Chung-Shan South Road, Taipei 100, Taiwan. *E-mail: [email protected] Accepted July 11, 2011. Amyloidosis refers to a variety of conditions in which a protein polysaccharide complex, amyloid protein, is accumulated locally or systemically in tissues or organs. Amyloid in the skin may be derived directly from kerati- nocytes, or secondarily from immunoglobulin light chain fragments (AL type), fragments of the acute-phase reac- tant serum amyloid A (AA type), etc. Nodular amyloido- sis (NA) is a rare type of localized AL amyloidosis, with most reported cases being of the lambda light chains (1) and is often histopathologically indistinguishable from systemic amyloidosis. Its association with lymphoma has been observed infrequently. CASE REPORT An 88-year-old woman initially noticed a 5×3 cm mass in her right breast in 1990. She was diagnosed with non-Hodgkin’s lymphoma (NHL) (diffuse large immunoblastic B-cell type, sta- ge IE) after the excision of the mass in a tertiary referral centre. Bone marrow biopsy revealed no evidence of lymphoma invol- vement. She achieved complete remission after chemotherapy. Six years after the diagnosis of lymphoma she developed multiple enlarging nodules on her legs. On examination, there were coalescing, waxy, skin-coloured, nodules with some foci of haemorrhage, central ulceration and crusts on the legs (Fig. -

Update on Hairy Cell Leukemia Robert J
Update on Hairy Cell Leukemia Robert J. Kreitman, MD, and Evgeny Arons, PhD The authors are affiliated with the Abstract: Hairy cell leukemia (HCL) is a chronic B-cell malignancy with National Cancer Institute’s Center for multiple treatment options, including several that are investigational. Cancer Research at the National Institutes Patients present with pancytopenia and splenomegaly, owing to the of Health in Bethesda, Maryland. Dr infiltration of leukemic cells expressing CD22, CD25, CD20, CD103, Kreitman is a senior investigator in the Laboratory of Molecular Biology and tartrate-resistant acid phosphatase (TRAP), annexin A1 (ANXA1), and the head of the clinical immunotherapy the BRAF V600E mutation. A variant lacking CD25, ANXA1, TRAP, section, and Dr Arons is a staff scientist in and the BRAF V600E mutation, called HCLv, is more aggressive and the Laboratory of Molecular Biology. is classified as a separate disease. A molecularly defined variant expressing unmutated immunoglobulin heavy variable 4-34 (IGHV4- 34) is also aggressive, lacks the BRAF V600E mutation, and has a Corresponding author: Robert J. Kreitman, MD phenotype of HCL or HCLv. The standard first-line treatment, which Laboratory of Molecular Biology has remained unchanged for the past 25 to 30 years, is single-agent National Cancer Institute therapy with a purine analogue, either cladribine or pentostatin. This National Institutes of Health approach produces a high rate of complete remission. Residual traces 9000 Rockville Pike of HCL cells, referred to as minimal residual disease, are present in Building 37, Room 5124b most patients and cause frequent relapse. Repeated treatment with Bethesda, MD 20892-4255 Tel: (301) 648-7375 a purine analogue can restore remission, but at decreasing rates and Fax: (301) 451-5765 with increasing cumulative toxicity. -

REVIEW Anti-CD20-Based Therapy of B Cell Lymphoma: State of The
Leukemia (2002) 16, 2004–2015 2002 Nature Publishing Group All rights reserved 0887-6924/02 $25.00 www.nature.com/leu REVIEW Anti-CD20-based therapy of B cell lymphoma: state of the art C Kosmas1, K Stamatopoulos2, N Stavroyianni2, N Tsavaris3 and T Papadaki4 1Department of Medicine, 2nd Division of Medical Oncology, ‘Metaxa’ Cancer Hospital, Piraeus, Greece; 2Department of Hematology, G Papanicolaou General Hospital, Thessaloniki, Greece; 3Oncology Unit, Department of Pathophysiology, Athens University School of Medicine, Laikon General Hospital, Athens, Greece; and 4Hemopathology Department, Evangelismos Hospital, Athens, Greece Over the last 5 years, studies applying the chimeric anti-CD20 ficulties in identifying a completely tumor-specific target; (2) MAb have renewed enthusiasm and triggered world-wide appli- the impracticality of constructing a unique antibody for each cation of anti-CD20 MAb-based therapies in B cell non-Hodg- kin’s lymphoma (NHL). Native chimeric anti-CD20 and isotope- patient; (3) the development of an immune response to murine 6 labeled murine anti-CD20 MAbs are currently employed with immunoglobulins (human anti-mouse antibodies, HAMA). By encouraging results as monotherapy or in combination with the end of the 1980s enthusiasm for therapeutic MAbs was conventional chemotherapy and in consolidation of remission waning; murine native (unconjugated), radioactively labeled after treatments with curative intent (ie after/ in combination or toxin-conjugated MAbs failed to yield significant clinical with high-dose chemotherapy and hematopoietic stem cell responses; moreover, they were not uncommonly associated rescue). On the available experience, anti-CD20 MAb-based therapeutic strategies will be increasingly integrated in the with toxicities, predominantly in the form of serum sickness treatment of B cell NHL and related malignancies. -

Molecular and Immunological Dissection of Diffuse Large
Leukemia (1999) 13, 1441–1447 1999 Stockton Press All rights reserved 0887-6924/99 $15.00 http://www.stockton-press.co.uk/leu ,Molecular and immunological dissection of diffuse large B cell lymphoma: CD5؉ and CD5؊ with CD10؉ groups may constitute clinically relevant subtypes S Harada1,2, R Suzuki1, K Uehira3, Y Yatabe4, Y Kagami3, M Ogura3, H Suzuki5, A Oyama6, Y Kodera7, R Ueda2, Y Morishima3, S Nakamura4 and M Seto1 1Laboratory of Chemotherapy, Aichi Cancer Center Research Institute; 3Department of Hematology and Chemotherapy, 4Department of Pathology and Clinical Laboratories, Aichi Cancer Center Hospital; 2Second Department of Internal Medicine, Nagoya City University School of Medicine; 5Department of Internal Medicine, Okazaki Municipal Hospital; 6Department of Internal Medicine, Aichi Prefectural Hospital; 7Department of Internal Medicine, Japanese Red Cross, Nagoya First Hospital, Japan Diffuse large B cell lymphoma (DLBL) constitutes the greatest genotypic parameters were found not to be helpful for deline- percentage of adult non-Hodgkin’s lymphomas and represents ating distinctive morphologic subgroups). DLBL accounts for a diverse spectrum of lymphoid neoplasms. Clinicopathologic, the greatest percentage (48%) of adult non-Hodgkin’s lym- phenotypic and genotypic findings were correlated and com- 4 pared for 63 DLBL cases to investigate whether they represent phoma (NHL) among Japanese patients and for about 40% clinically relevant subtypes. They were all cyclin D1 negative of initial NHL diagnoses in the United States.5 Among DLBL, and were phenotypically divided into three groups, ie group I only a few entities, eg primary mediastinal large B cell lym- + − + (CD5 type, n = 11), group II (CD5 CD10 type, n = 19), and phoma characterized by the fact that it has a peculiar disease − − = group III (CD5 CD10 type, n 33).