The Distinction Between Burkitt Lymphoma and Diffuse Large B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Cutaneous Anaplastic Large Cell Lymphoma / Lymphomatoid
Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders Definition A spectrum of related conditions originating from transformed or activated CD30-positive T-lymphocytes May coexist in individual patients Clonally related Overlapping clinical and/or histological features Clinical, histologic, and phenotypic characteristics required for diagnosis Types 1. Primary cutaneous anaplastic large cell lymphoma (C-ALCL) 2. Lymphomatoid papulosis 3. Borderline lesions C-ALCL: Definition T-cell lymphoma, presenting in the skin and consisting of anaplastic lymphoid cells, the majority of which are CD30- positive Distinction from: (a) systemic ALCL with cutaneous involvement, and (b) secondary high-grade lymphomas with CD30 expression In nearly all patients disease is limited to the skin at the time of diagnosis Assessed by meticulous staging Patients should not have other subtypes of lymphoma C-ALCL: Synonyms Lukes-Collins: Not listed (T-immunoblastic) Kiel: Anaplastic large cell Working Formulation: Various categories (diffuse large cell; immunoblastic) REAL: Primary cutaneous anaplastic large cell (CD30+) lymphoma Related terms: Regressing atypical histiocytosis; Ki-1 lymphoma C-ALCL: Epidemiology 25% of the T-cell lymphomas arising primarily in the skin. Predominantly in adults/elderly and rare in children. The male to female ratio is 1.5-2.0:1. C-ALCL: Sites of Involvement The disease is nearly always limited to the skin at the time of diagnosis Extracutaneous dissemination may occur Mainly regional lymph nodes Involvement of other organs is rare C-ALCL: Clinical Features Most present solitary or localized skin lesions which may be tumors, nodules or (more rarely) papules Multicentric cutaneous disease occurs in 20% Lesions may show partial or complete spontaneous regression (similar to lymphomatoid papulosis) Cutaneous relapses are frequent Extracutaneous dissemination occurs in approximately 10% of the patients. -

Quantitative Analysis of Bcl-2 Expression in Normal and Leukemic Human B-Cell Differentiation
Leukemia (2004) 18, 491–498 & 2004 Nature Publishing Group All rights reserved 0887-6924/04 $25.00 www.nature.com/leu Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation P Menendez1,2, A Vargas3, C Bueno1,2, S Barrena1,2, J Almeida1,2 M de Santiago1,2,ALo´pez1,2, S Roa2, JF San Miguel2,4 and A Orfao1,2 1Servicio General de Citometrı´a, Universidad de Salamanca, Salamanca, Spain; 2Departamento de Medicina and Centro de Investigacio´n del Ca´ncer, Universidad de Salamanca, Salamanca, Spain; 3Servicio de Inmunodiagno´stico, Departamento de Patologı´a Clı´nica, Hospital Nacional Edgardo Rebagliati Martins, Lima, Peru´, ; and 4Servicio de Hematologı´a, Hospital Universitario, Salamanca, Spain Lack of apoptosis has been linked to prolonged survival of results in the overexpression of the bcl-2 protein and it malignant B cells expressing bcl-2. The aim of the present represented the first clear example of a common step in study was to analyze the amount of bcl-2 protein expressed 9,10 along normal human B-cell maturation and to establish the oncogenesis mediated by decreased cell death. Currently, frequency of aberrant bcl-2 expression in B-cell malignancies. the exact antiapoptotic pathways through which bcl-2 exerts its In normal bone marrow (n ¼ 11), bcl-2 expression obtained by role are only partially understood, involving decreased mito- quantitative multiparametric flow cytometry was highly vari- chondrial release of cytochrome c, which in turn is required for þ À able: very low in both CD34 and CD34 B-cell precursors, high the activation of procaspase-9 and the subsequent initiation of in mature B-lymphocytes and very high in plasma cells. -

Hematopoietic and Lymphoid Neoplasm Coding Manual
Hematopoietic and Lymphoid Neoplasm Coding Manual Effective with Cases Diagnosed 1/1/2010 and Forward Published August 2021 Editors: Jennifer Ruhl, MSHCA, RHIT, CCS, CTR, NCI SEER Margaret (Peggy) Adamo, BS, AAS, RHIT, CTR, NCI SEER Lois Dickie, CTR, NCI SEER Serban Negoita, MD, PhD, CTR, NCI SEER Suggested citation: Ruhl J, Adamo M, Dickie L., Negoita, S. (August 2021). Hematopoietic and Lymphoid Neoplasm Coding Manual. National Cancer Institute, Bethesda, MD, 2021. Hematopoietic and Lymphoid Neoplasm Coding Manual 1 In Appreciation NCI SEER gratefully acknowledges the dedicated work of Drs, Charles Platz and Graca Dores since the inception of the Hematopoietic project. They continue to provide support. We deeply appreciate their willingness to serve as advisors for the rules within this manual. The quality of this Hematopoietic project is directly related to their commitment. NCI SEER would also like to acknowledge the following individuals who provided input on the manual and/or the database. Their contributions are greatly appreciated. • Carolyn Callaghan, CTR (SEER Seattle Registry) • Tiffany Janes, CTR (SEER Seattle Registry) We would also like to give a special thanks to the following individuals at Information Management Services, Inc. (IMS) who provide us with document support and web development. • Suzanne Adams, BS, CTR • Ginger Carter, BA • Sean Brennan, BS • Paul Stephenson, BS • Jacob Tomlinson, BS Hematopoietic and Lymphoid Neoplasm Coding Manual 2 Dedication The Hematopoietic and Lymphoid Neoplasm Coding Manual (Heme manual) and the companion Hematopoietic and Lymphoid Neoplasm Database (Heme DB) are dedicated to the hard-working cancer registrars across the world who meticulously identify, abstract, and code cancer data. -

SNF Mobility Model: ICD-10 HCC Crosswalk, V. 3.0.1
The mapping below corresponds to NQF #2634 and NQF #2636. HCC # ICD-10 Code ICD-10 Code Category This is a filter ceThis is a filter cellThis is a filter cell 3 A0101 Typhoid meningitis 3 A0221 Salmonella meningitis 3 A066 Amebic brain abscess 3 A170 Tuberculous meningitis 3 A171 Meningeal tuberculoma 3 A1781 Tuberculoma of brain and spinal cord 3 A1782 Tuberculous meningoencephalitis 3 A1783 Tuberculous neuritis 3 A1789 Other tuberculosis of nervous system 3 A179 Tuberculosis of nervous system, unspecified 3 A203 Plague meningitis 3 A2781 Aseptic meningitis in leptospirosis 3 A3211 Listerial meningitis 3 A3212 Listerial meningoencephalitis 3 A34 Obstetrical tetanus 3 A35 Other tetanus 3 A390 Meningococcal meningitis 3 A3981 Meningococcal encephalitis 3 A4281 Actinomycotic meningitis 3 A4282 Actinomycotic encephalitis 3 A5040 Late congenital neurosyphilis, unspecified 3 A5041 Late congenital syphilitic meningitis 3 A5042 Late congenital syphilitic encephalitis 3 A5043 Late congenital syphilitic polyneuropathy 3 A5044 Late congenital syphilitic optic nerve atrophy 3 A5045 Juvenile general paresis 3 A5049 Other late congenital neurosyphilis 3 A5141 Secondary syphilitic meningitis 3 A5210 Symptomatic neurosyphilis, unspecified 3 A5211 Tabes dorsalis 3 A5212 Other cerebrospinal syphilis 3 A5213 Late syphilitic meningitis 3 A5214 Late syphilitic encephalitis 3 A5215 Late syphilitic neuropathy 3 A5216 Charcot's arthropathy (tabetic) 3 A5217 General paresis 3 A5219 Other symptomatic neurosyphilis 3 A522 Asymptomatic neurosyphilis 3 A523 Neurosyphilis, -

Indolent Non-Hodgkin's Lymphomas
Follicular and Low-Grade Non-Hodgkin Lymphomas (Indolent Lymphomas) Stefan K Barta, M.D., M.S. Associate Professor of Medicine Leader, T Cell Lymphoma Program Perelman Center for Advanced Medicine Facts and Figures: Non-Hodgkin Lymphomas • Most common blood cancer • 7th most common cancer in the US3 • 71,850 new cases in the US in 20151 • 19,790 died of NHL in 20151 • About 549,625 people are living with a history of NHL (2012)1 • 85% of all NHLs are B-cell lymphomas2 • Follicular lymphoma = 2nd most common type, ~25% of all NHLs4 1 http://seer.cancer.gov/statfacts/html/nhl.html. 2 ACS. Detailed Guide (revised January 21, 2000): Non-Hodgkin’s Lymphoma. 3 http://www.cancer.gov/cancertopics/types/commoncancers 4 Blood 89: 3909, 1997 The Immune System T- CELLS B- CELLS Cellular immunity: Humoral immunity: helper + cytotoxic T-cells antibodies Lymphatic System Lymph Node Anatomy Lymph Node: Microscopic View germinal center Lymphocyte: Microscopic View Causes Possible cause(s): • chemical exposures (pesticides, fertilizers or solvents) • individuals with compromised immune systems • heredity • infections (e.g. H. pylori, Hep C, chlamydia trachomatis) • most patients have no clear risk factors • IN MOST CASES, THE EXACT CAUSE IS UNKNOWN Cellular Origins of Lymphomas & Leukemias PLEURIPOTENT STEM CELL ACUTE LEUKEMIAS LYMPHOID STEM CELL ACUTE LYMPHOBLASTIC LEUKEMIAS PRECURSOR T - CELL PRECURSOR B - CELL LYMPHOBLASTIC LYMPHOMAS / LEUKEMIAS MATURE T - CELL MATURE B - CELL NON-HODGKIN LYMPHOMAS / CHRONIC LYMPHOCYTIC LEUKEMIA LYMPH NODES, EXTRANODAL -

Lymphoproliferative Disorders
Lymphoproliferative disorders Objectives: • To understand the general features of lymphoproliferative disorders (LPD) • To understand some benign causes of LPD such as infectious mononucleosis • To understand the general classification of malignant LPD Important. • To understand the clinicopathological features of chronic lymphoid leukemia Extra. • To understand the general features of the most common Notes (LPD) (Burkitt lymphoma, Follicular • lymphoma, multiple myeloma and Hodgkin lymphoma). Success is the result of perfection, hard work, learning Powellfrom failure, loyalty, and persistence. Colin References: Editing file 435 teamwork slides 6 girls & boys slides Do you have any suggestions? Please contact us! @haematology436 E-mail: [email protected] or simply use this form Definitions Lymphoma (20min) Lymphoproliferative disorders: Several clinical conditions in which lymphocytes are produced in excessive quantities (Lymphocytosis) increase in lymphocytes that are not normal Lymphoma: Malignant lymphoid mass involving the lymphoid tissues. (± other tissues e.g: skin, GIT, CNS ..) The main deference between Lymphoma & Leukemia is that the Lymphoma proliferate primarily in Lymphoid Tissue and cause Mass , While Leukemia proliferate mainly in BM& Peripheral blood Lymphoid leukemia: Malignant proliferation of lymphoid cells in Bone marrow and peripheral blood. (± other tissues e.g: lymph nodes, spleen, skin, GIT, CNS ..) BCL is an anti-apoptotic (prevent apoptosis) Lymphocytosis (causes) 1- Viral infection: 2- Some* bacterial -

Non-Hodgkin and Hodgkin Lymphomas Select for Overexpression of BCLW Clare M
Published OnlineFirst August 29, 2017; DOI: 10.1158/1078-0432.CCR-17-1144 Biology of Human Tumors Clinical Cancer Research Non-Hodgkin and Hodgkin Lymphomas Select for Overexpression of BCLW Clare M. Adams1, Ramkrishna Mitra1, Jerald Z. Gong2, and Christine M. Eischen1 Abstract Purpose: B-cell lymphomas must acquire resistance to apopto- follicular, mantle cell, marginal zone, and Hodgkin lymphomas. sis during their development. We recently discovered BCLW, an Notably, BCLW was preferentially overexpressed over that of antiapoptotic BCL2 family member thought only to contribute to BCL2 and negatively correlated with BCL2 in specific lymphomas. spermatogenesis, was overexpressed in diffuse large B-cell lym- Unexpectedly, BCLW was overexpressed as frequently as BCL2 in phoma (DLBCL) and Burkitt lymphoma. To gain insight into the follicular lymphoma. Evaluation of all five antiapoptotic BCL2 contribution of BCLW to B-cell lymphomas and its potential to family members in six types of B-cell lymphoma revealed that confer resistance to BCL2 inhibitors, we investigated the expres- BCL2, BCLW, and BCLX were consistently overexpressed, whereas sion of BCLW and the other antiapoptotic BCL2 family members MCL1 and A1 were not. In addition, individual lymphomas in six different B-cell lymphomas. frequently overexpressed more than one antiapoptotic BCL2 Experimental Design: We performed a large-scale gene family member. expression analysis of datasets comprising approximately Conclusions: Our comprehensive analysis indicates B-cell 2,300 lymphoma patient samples, including non-Hodgkin lymphomas commonly select for BCLW overexpression in andHodgkinlymphomasaswellasindolentandaggressive combination with or instead of other antiapoptotic BCL2 lymphomas. Data were validated experimentally with qRT- family members. Our results suggest BCLW may be equally as PCR and IHC. -

Non-Hodgkin Lymphoma
Non-Hodgkin Lymphoma Rick, non-Hodgkin lymphoma survivor This publication was supported in part by grants from Revised 2013 A Message From John Walter President and CEO of The Leukemia & Lymphoma Society The Leukemia & Lymphoma Society (LLS) believes we are living at an extraordinary moment. LLS is committed to bringing you the most up-to-date blood cancer information. We know how important it is for you to have an accurate understanding of your diagnosis, treatment and support options. An important part of our mission is bringing you the latest information about advances in treatment for non-Hodgkin lymphoma, so you can work with your healthcare team to determine the best options for the best outcomes. Our vision is that one day the great majority of people who have been diagnosed with non-Hodgkin lymphoma will be cured or will be able to manage their disease with a good quality of life. We hope that the information in this publication will help you along your journey. LLS is the world’s largest voluntary health organization dedicated to funding blood cancer research, education and patient services. Since 1954, LLS has been a driving force behind almost every treatment breakthrough for patients with blood cancers, and we have awarded almost $1 billion to fund blood cancer research. Our commitment to pioneering science has contributed to an unprecedented rise in survival rates for people with many different blood cancers. Until there is a cure, LLS will continue to invest in research, patient support programs and services that improve the quality of life for patients and families. -
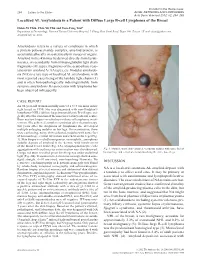
Localized AL Amyloidosis in a Patient with Diffuse Large B-Cell Lymphoma of the Breast
Included in the theme issue: 284 Letters to the Editor ACNE, RETINOIDS AND LYMPHOMAS Acta Derm Venereol 2012; 92: 284–285 Localized AL Amyloidosis in a Patient with Diffuse Large B-cell Lymphoma of the Breast Hsien-Yi Chiu, Chia-Yu Chu and Tsen-Fang Tsai* Department of Dermatology, National Taiwan University Hospital, 7 Chung-Shan South Road, Taipei 100, Taiwan. *E-mail: [email protected] Accepted July 11, 2011. Amyloidosis refers to a variety of conditions in which a protein polysaccharide complex, amyloid protein, is accumulated locally or systemically in tissues or organs. Amyloid in the skin may be derived directly from kerati- nocytes, or secondarily from immunoglobulin light chain fragments (AL type), fragments of the acute-phase reac- tant serum amyloid A (AA type), etc. Nodular amyloido- sis (NA) is a rare type of localized AL amyloidosis, with most reported cases being of the lambda light chains (1) and is often histopathologically indistinguishable from systemic amyloidosis. Its association with lymphoma has been observed infrequently. CASE REPORT An 88-year-old woman initially noticed a 5×3 cm mass in her right breast in 1990. She was diagnosed with non-Hodgkin’s lymphoma (NHL) (diffuse large immunoblastic B-cell type, sta- ge IE) after the excision of the mass in a tertiary referral centre. Bone marrow biopsy revealed no evidence of lymphoma invol- vement. She achieved complete remission after chemotherapy. Six years after the diagnosis of lymphoma she developed multiple enlarging nodules on her legs. On examination, there were coalescing, waxy, skin-coloured, nodules with some foci of haemorrhage, central ulceration and crusts on the legs (Fig. -

Mature B-Cell Neoplasms
PEARLS OF LABORATORY MEDICINE Mature B-cell Neoplasms Michael Moravek, MD Kamran M. Mirza, MD, PhD Loyola University Chicago Stritch School of Medicine DOI: 10.15428/CCTC.2018.287706 © Clinical Chemistry The Lymphoid System Bone Marrow Stem Cell • Mature B-cell lymphomas comprise approximately 75% of all lymphoid neoplasms • Genetic alterations lead to deregulation of cell T cell Immature NK cell proliferation or apoptosis B cell • Low-grade lymphomas typically present as painless B cell lymphadenopathy, hepatosplenomegaly, or incidental lymphocytosis • High-grade lymphomas typically present with a rapidly enlarging mass and “B” symptoms (fever, weight loss, night sweats) Plasma cell 2 B-Cell Maturation antigen Naïve B cell Post- Germinal Center Germinal Center Marginal Zone DLBCL (some) FL, BL, some DLBCL, CLL/SLL Mantle Cell Hodgkin lymphoma MZL MALT Lymphoma Plasma cell myeloma CD5 + CD10 + CD5/CD10 Neg 3 Frequency among mature B-cell neoplasms DLBCL 27.6% CLL/SLL 19.4% Mature B-cell neoplasms Chronic lymphocytic leukemia/small lymphocytic lymphoma Primary cutaneous follicle center lymphoma Follicular Lymphoma -Monoclonal B-12.2%cell lymphocytosis Mantle cell lymphoma Marginal Zone LymphomaB-cell prolymphocytic 3.7% leukemia -Leukemic non-nodal mantle cell lymphoma Splenic marginal zone lymphoma -In situ mantle cell neoplasia Mantle Cell LymphomaHairy cell leukemia1.9% Diffuse large B-cell lymphoma (DLBCL), NOS Splenic B-cell lymphoma/leukemia, unclassifiable -Germinal center B-cell type Burkitt Lymphoma -Splenic diffuse1.3% red pulp -

Burkitt Lymphoma
Board Review- Part 2B: Malignant HemePath 4/25/2018 Small Lymphocytic Lymphoma SLL: epidemiology SLL: 6.7% of non-Hodgkin lymphoma. Majority of patients >50 y/o (median 65). M:F ratio 2:1. Morphology • Lymph nodes – Effacement of architecture, pseudofollicular pattern of pale areas of large cells in a dark background of small cells. Occasionally is interfollicular. – The predominant cell is a small lymphocyte with clumped chromatin, round nucleus, ocassionally a nucleolus. – Mitotic activity usually very low. Morphology - Pseudofollicles or proliferation centers contain small, medium and large cells. - Prolymphocytes are medium-sized with dispersed chromatin and small nucleoli. - Paraimmunoblasts are medium to large cells with round to oval nuclei, dispersed chromatin, central eosinophilic nucleoli and slightly basophilic cytoplasm. Small Lymphocytic Lymphoma Pseudo-follicle Small Lymphocytic Lymphoma Prolymphocyte Paraimmunoblast Immunophenotype Express weak or dim surface IgM or IgM and IgD, CD5, CD19, CD20 (weak), CD22 (weak), CD79a, CD23, CD43, CD11c (weak). CD10-, cyclin D1-. FMC7 and CD79b negative or weak. Immunophenotype Cases with unmutated Ig variable region genes are reported to be CD38+ and ZAP70+. Immunophenotype Cytoplasmic Ig is detectable in about 5% of the cases. CD5 and CD23 are useful in distinguishing from MCL. Rarely CLL is CD23-. Rarely MCL is CD23+. Perform Cyclin D1 in CD5+/CD23- cases. Some cases with typical CLL morphology may have a different profile (CD5- or CD23-, FMC7+ or CD11c+, or strong sIg, or CD79b+). Genetics Antigen receptor genes: Ig heavy and light chain genes are rearranged. Suggestion of 2 distinct types of SLL defined by the mutational status of the IgVH genes: 40-50% show no somatic mutations of their variable region genes (naïve cells, unmutated). -

REVIEW Anti-CD20-Based Therapy of B Cell Lymphoma: State of The
Leukemia (2002) 16, 2004–2015 2002 Nature Publishing Group All rights reserved 0887-6924/02 $25.00 www.nature.com/leu REVIEW Anti-CD20-based therapy of B cell lymphoma: state of the art C Kosmas1, K Stamatopoulos2, N Stavroyianni2, N Tsavaris3 and T Papadaki4 1Department of Medicine, 2nd Division of Medical Oncology, ‘Metaxa’ Cancer Hospital, Piraeus, Greece; 2Department of Hematology, G Papanicolaou General Hospital, Thessaloniki, Greece; 3Oncology Unit, Department of Pathophysiology, Athens University School of Medicine, Laikon General Hospital, Athens, Greece; and 4Hemopathology Department, Evangelismos Hospital, Athens, Greece Over the last 5 years, studies applying the chimeric anti-CD20 ficulties in identifying a completely tumor-specific target; (2) MAb have renewed enthusiasm and triggered world-wide appli- the impracticality of constructing a unique antibody for each cation of anti-CD20 MAb-based therapies in B cell non-Hodg- kin’s lymphoma (NHL). Native chimeric anti-CD20 and isotope- patient; (3) the development of an immune response to murine 6 labeled murine anti-CD20 MAbs are currently employed with immunoglobulins (human anti-mouse antibodies, HAMA). By encouraging results as monotherapy or in combination with the end of the 1980s enthusiasm for therapeutic MAbs was conventional chemotherapy and in consolidation of remission waning; murine native (unconjugated), radioactively labeled after treatments with curative intent (ie after/ in combination or toxin-conjugated MAbs failed to yield significant clinical with high-dose chemotherapy and hematopoietic stem cell responses; moreover, they were not uncommonly associated rescue). On the available experience, anti-CD20 MAb-based therapeutic strategies will be increasingly integrated in the with toxicities, predominantly in the form of serum sickness treatment of B cell NHL and related malignancies.