A Transcriptomic Analysis of Diploid and Triploid Atlantic Salmon Lenses with and Without Cataracts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Browsing Genes and Genomes with Ensembl
The Bioinformatics Roadshow Tórshavn, The Faroe Islands 28-29 November 2012 BROWSING GENES AND GENOMES WITH ENSEMBL EXERCISES AND ANSWERS 1 BROWSER 3 BIOMART 8 VARIATION 13 COMPARATIVE GENOMICS 18 2 Note: These exercises are based on Ensembl version 69 (October 2012). After in future a new version has gone live, version 69 will still be available at http://e69.ensembl.org/. If your answer doesn’t correspond with the given answer, please consult the instructor. ______________________________________________________________ BROWSER ______________________________________________________________ Exercise 1 – Exploring a gene (a) Find the human F9 (coagulation factor IX) gene. On which chromosome and which strand of the genome is this gene located? How many transcripts (splice variants) have been annotated for it? (b) What is the longest transcript? How long is the protein it encodes? Has this transcript been annotated automatically (by Ensembl) or manually (by Havana)? How many exons does it have? Are any of the exons completely or partially untranslated? (c) Have a look at the external references for ENST00000218099. What is the function of F9? (d) Is it possible to monitor expression of ENST00000218099 with the ILLUMINA HumanWG_6_V2 microarray? If so, can it also be used to monitor expression of the other two transcripts? (e) In which part (i.e. the N-terminal or C-terminal half) of the protein encoded by ENST00000218099 does its peptidase activity reside? (f) Have any missense variants been discovered for the protein encoded by ENST00000218099? (g) Is there a mouse orthologue predicted for the human F9 gene? (h) If you have yourself a gene of interest, explore what information Ensembl displays about it! ______________________________________________________________ Answer (a) 8 Go to the Ensembl homepage (http://www.ensembl.org/). -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T (51) International Patent Classification: David [US/US]; 13539 N . 95th Way, Scottsdale, AZ C12Q 1/68 (2006.01) 85260 (US). (21) International Application Number: (74) Agent: AKHAVAN, Ramin; Caris Science, Inc., 6655 N . PCT/US20 12/0425 19 Macarthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 14 June 2012 (14.06.2012) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, English (25) Filing Language: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, Publication Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/497,895 16 June 201 1 (16.06.201 1) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 61/499,138 20 June 201 1 (20.06.201 1) US OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 61/501,680 27 June 201 1 (27.06.201 1) u s SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 61/506,019 8 July 201 1(08.07.201 1) u s TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Definition of the Landscape of Promoter DNA Hypomethylation in Liver Cancer
Published OnlineFirst July 11, 2011; DOI: 10.1158/0008-5472.CAN-10-3823 Cancer Therapeutics, Targets, and Chemical Biology Research Definition of the Landscape of Promoter DNA Hypomethylation in Liver Cancer Barbara Stefanska1, Jian Huang4, Bishnu Bhattacharyya1, Matthew Suderman1,2, Michael Hallett3, Ze-Guang Han4, and Moshe Szyf1,2 Abstract We use hepatic cellular carcinoma (HCC), one of the most common human cancers, as a model to delineate the landscape of promoter hypomethylation in cancer. Using a combination of methylated DNA immunopre- cipitation and hybridization with comprehensive promoter arrays, we have identified approximately 3,700 promoters that are hypomethylated in tumor samples. The hypomethylated promoters appeared in clusters across the genome suggesting that a high-level organization underlies the epigenomic changes in cancer. In normal liver, most hypomethylated promoters showed an intermediate level of methylation and expression, however, high-CpG dense promoters showed the most profound increase in gene expression. The demethylated genes are mainly involved in cell growth, cell adhesion and communication, signal transduction, mobility, and invasion; functions that are essential for cancer progression and metastasis. The DNA methylation inhibitor, 5- aza-20-deoxycytidine, activated several of the genes that are demethylated and induced in tumors, supporting a causal role for demethylation in activation of these genes. Previous studies suggested that MBD2 was involved in demethylation of specific human breast and prostate cancer genes. Whereas MBD2 depletion in normal liver cells had little or no effect, we found that its depletion in human HCC and adenocarcinoma cells resulted in suppression of cell growth, anchorage-independent growth and invasiveness as well as an increase in promoter methylation and silencing of several of the genes that are hypomethylated in tumors. -
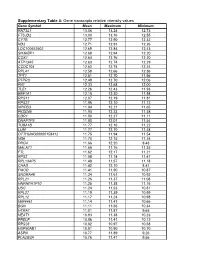
Gene Transcripts Relative Intensity Values
Supplementary Table 4: Gene transcripts relative intensity values Gene Symbol Mean Maximum Minimum RN7SL1 13.06 13.24 12.73 FTSJD2 13.00 13.16 12.55 CYTB 12.77 12.90 12.32 ND2 12.71 12.91 12.26 LOC100652902 12.69 12.84 12.43 SH3KBP1 12.68 12.94 12.20 COX1 12.64 12.76 12.20 ATP13A5 12.63 12.74 12.29 CCDC104 12.62 12.74 12.24 RPL41 12.58 12.66 12.36 TPT1 12.51 12.70 11.96 PTPRO 12.49 12.70 12.06 FN1 12.33 12.68 12.00 TLE1 12.26 12.43 11.93 EEF1A1 12.15 12.30 11.98 RPS11 12.07 12.19 11.81 RPS27 11.96 12.10 11.72 NPIPB3 11.94 12.21 11.65 FKSG49 11.94 12.23 11.58 CDR1 11.90 12.27 11.11 DNAPTP3 11.80 12.07 11.54 TUBA1B 11.77 12.16 11.22 LUM 11.77 12.10 11.48 OTTHUMG00000158412 11.75 11.94 11.54 ND6 11.70 12.15 11.24 PRG4 11.66 12.20 9.43 MALAT1 11.65 11.76 11.35 FTL 11.62 12.17 11.21 RPS2 11.58 11.74 11.47 RPL13AP5 11.48 11.57 11.18 CHAD 11.42 12.10 8.41 FMOD 11.41 11.90 10.87 SNORA48 11.34 11.61 10.92 RPL21 11.26 11.37 11.08 HNRNPA1P10 11.25 11.38 11.16 UBC 11.24 11.53 10.81 RPL27 11.19 11.39 10.99 RPL12 11.17 11.24 10.98 MIR4461 11.14 11.47 10.66 BGN 11.11 11.66 10.34 HTRA1 11.01 11.87 9.65 NEAT1 10.93 11.38 10.23 PRELP 10.86 11.41 10.12 RPS28 10.82 10.97 10.58 HSP90AB1 10.81 10.90 10.70 ASPN 10.77 11.89 9.26 PLA2G2A 10.76 11.47 9.56 H3F3A 10.69 10.86 10.41 EEF1G 10.69 10.94 10.11 C6orf48 10.64 11.02 10.35 UBA52 10.58 10.75 10.08 CTGF 10.57 11.33 9.66 MGP 10.57 11.22 10.09 YBX1 10.56 10.79 10.01 MT1X 10.56 11.89 9.88 NPC2 10.55 10.80 10.12 LAPTM4A 10.51 10.78 10.18 ITM2B 10.51 10.68 10.34 IBSP 10.50 11.50 7.17 NPIPB5 10.50 10.73 10.26 TUBA1A -

Brain Deletion of Serf2 Shifts Amyloid Conformation in a Mouse Model
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.05.423442; this version posted March 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Brain deletion of Serf2 shifts amyloid conformation in a mouse model 2 E. Stroo1#, L. Janssen1#*, O. Sin1, W. Hogewerf1, M. Koster2, L. Harkema2, S.A. Youssef2,3, N. 3 Beschorner4, A.H.G. Wolters5, B. Bakker1, A. Thathiah6,7, F. Foijer1, B. van de Sluis2, J. van Deursen8, 4 M. Jucker4, A. de Bruin2,3, E.A.A. Nollen1* 5 1European Research Institute for the Biology of Ageing, University of Groningen, University Medical 6 Centre Groningen, The Netherlands 7 2Department of Biomolecular Health Sciences, Faculty of Veterinary Medicine, Utrecht University, 8 The Netherlands 9 3Department of Pediatrics, Molecular Genetics Section, University of Groningen, University Medical 10 Centre Groningen, The Netherlands 11 4Department of Cellular Neurology, Hertie-Institute for Clinical Brain Research, University of 12 Tübingen, Tübingen, Germany. 13 5Department of Biomedical Sciences of Cells and Systems, University Medical Centre Groningen, The 14 Netherlands 15 6VIB Center for the Biology of Disease, KU Leuven Center for Human Genetics, University 16 of Leuven, Leuven, Belgium 17 7 Department of Neurobiology, University of Pittsburgh Brain Institute, University of 18 Pittsburgh School of Medicine, Pittsburgh, PA USA 19 8Mayo Clinic, United States of America 20 #These authors contributed equally 21 *Corresponding authors: [email protected] or [email protected] 22 Keywords: Small EDRK-rich factor 2, SERF, protein aggregation, amyloid-beta, amyloid 23 polymorphisms bioRxiv preprint doi: https://doi.org/10.1101/2021.01.05.423442; this version posted March 3, 2021. -

Robles JTO Supplemental Digital Content 1
Supplementary Materials An Integrated Prognostic Classifier for Stage I Lung Adenocarcinoma based on mRNA, microRNA and DNA Methylation Biomarkers Ana I. Robles1, Eri Arai2, Ewy A. Mathé1, Hirokazu Okayama1, Aaron Schetter1, Derek Brown1, David Petersen3, Elise D. Bowman1, Rintaro Noro1, Judith A. Welsh1, Daniel C. Edelman3, Holly S. Stevenson3, Yonghong Wang3, Naoto Tsuchiya4, Takashi Kohno4, Vidar Skaug5, Steen Mollerup5, Aage Haugen5, Paul S. Meltzer3, Jun Yokota6, Yae Kanai2 and Curtis C. Harris1 Affiliations: 1Laboratory of Human Carcinogenesis, NCI-CCR, National Institutes of Health, Bethesda, MD 20892, USA. 2Division of Molecular Pathology, National Cancer Center Research Institute, Tokyo 104-0045, Japan. 3Genetics Branch, NCI-CCR, National Institutes of Health, Bethesda, MD 20892, USA. 4Division of Genome Biology, National Cancer Center Research Institute, Tokyo 104-0045, Japan. 5Department of Chemical and Biological Working Environment, National Institute of Occupational Health, NO-0033 Oslo, Norway. 6Genomics and Epigenomics of Cancer Prediction Program, Institute of Predictive and Personalized Medicine of Cancer (IMPPC), 08916 Badalona (Barcelona), Spain. List of Supplementary Materials Supplementary Materials and Methods Fig. S1. Hierarchical clustering of based on CpG sites differentially-methylated in Stage I ADC compared to non-tumor adjacent tissues. Fig. S2. Confirmatory pyrosequencing analysis of DNA methylation at the HOXA9 locus in Stage I ADC from a subset of the NCI microarray cohort. 1 Fig. S3. Methylation Beta-values for HOXA9 probe cg26521404 in Stage I ADC samples from Japan. Fig. S4. Kaplan-Meier analysis of HOXA9 promoter methylation in a published cohort of Stage I lung ADC (J Clin Oncol 2013;31(32):4140-7). Fig. S5. Kaplan-Meier analysis of a combined prognostic biomarker in Stage I lung ADC. -

This Item Is the Archived Peer-Reviewed Author-Version Of
This item is the archived peer-reviewed author-version of: Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Reference: Houbrechts Anne M., Vergauwen Lucia, Bagci Enise, Van houcke Jolien, Heijlen Marjolein, Kulemeka Bernard, Hyde David R., Knapen Dries, Darras Veerle M..- Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Molecular and cellular endocrinology - ISSN 0303-7207 - 424(2016), p. 81-93 Full text (Publishers DOI): http://dx.doi.org/doi:10.1016/j.mce.2016.01.018 To cite this reference: http://hdl.handle.net/10067/1314550151162165141 Institutional repository IRUA 1 Deiodinase knockdown affects zebrafish eye development at the level 2 of gene expression, morphology and function. 3 Anne M. Houbrechts1*, Lucia Vergauwen2*, Enise Bagci2,3, Jolien Van houcke1, Marjolein 4 Heijlen1, Bernard Kulemeka4, David R. Hyde4, Dries Knapen2, Veerle M. Darras1 5 1 Laboratory of Comparative Endocrinology, Department of Biology, Division of 6 Animal Physiology and Neurobiology, KU Leuven, 3000 Leuven, Belgium 7 2 Zebrafishlab, Veterinary Physiology and Biochemistry, Department of Veterinary 8 Sciences, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 9 3 Systemic Physiological & Ecotoxicological Research, Department of Biology, 10 University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 11 4 Department of Biological Sciences and Center for Zebrafish Research, University of 12 Notre Dame, Notre Dame, -

(SERF2) Promoter by Heat Shock Factor 1
Biochemistry and Biophysics Reports 7 (2016) 188–194 Contents lists available at ScienceDirect Biochemistry and Biophysics Reports journal homepage: www.elsevier.com/locate/bbrep Trans-activation of small EDRK-rich factor 2 (SERF2) promoter by Heat Shock Factor 1 Srijit Das a, Nitai Pada Bhattacharyya a,b,n a Crystallography & Molecular Biology Division, Saha Institute of Nuclear Physics, 1/AF Bidhannagar, Kolkata 700064, India b Biomedical Genomics Centre, PG Polyclinic Building (3rd floor), 5, Suburbun Hospital Road, Kolkata 700020, India article info abstract Article history: Heat shock response is an adaptive mechanism of cells characterized by rapid synthesis of a class of Received 25 July 2015 proteins popularly known as heat shock proteins (HSPs) by heat-induced activation of Heat Shock Factor Received in revised form 1 (HSF1). In course of our earlier study to show that HSF1 regulates transcription of HYPK (Huntingtin 30 March 2016 Yeast two-hybrid protein K), a chaperone-like protein, we observed presence of few other genes within Accepted 6 April 2016 10 kb of HYPK promoter. In an attempt to understand whether adjacent genes of HYPK are co-regulated, Available online 7 April 2016 we identified that SERF2 (small EDRK-rich factor 2), an upstream neighboring gene of HYPK, is also Keywords: regulated by heat stress and HSF1. We also showed that SERF2 promoter can be trans-activated by HSF1 Conjoined Gene due to the presence of functional heat shock element (HSE). Strikingly, HYPK is linked with SERF2 through small EDRK-rich factor 2 a Conjoined Gene (CG) albeit the respective proteins have opposite effect on mutant Huntingtin ag- Huntingtin Yeast-two-hybrid protein K gregates and subsequent toxicity. -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2020; 24: 1877-1886 Identification of lung adenocarcinoma-specific exosome RNAs in peripheral blood by RNA-Seq analysis X.-Q. LIU1, A. TUFMAN1, R. KIEFL1, G.-F. LI2, Q.-L. MA2, R.M. HUBER1 1Department of Internal Medicine V, Division of Respiratory Medicine and Thoracic Oncology, Ludwig-Maximilians University (LMU), Thoracic Oncology Centre Munich; Comprehensive Pneumology Center Munich, Member of the German Center for Lung Research (DZL), Munich, Bavaria, Germany 2Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Center. Kunming, Yunnan, China Abstract. – OBJECTIVE: Several plasma-de- carcinoma patients, who were more abundant/ rived exosome RNAs have been identified as key detectable than healthy volunteers. regulators in cancer development. They have CONCLUSIONS: Our data indicate that been considered as potential biomarkers for a plasma-derived exosome RNAs, UGT1A1, non-invasive “liquid biopsy” to diagnose and and BAIAP2L1, as well as the eight isolated assess the progression of cancer. This study pseudogenes could serve as diagnostic and aimed to identify human lung adenocarcino- prognostic biomarkers for an effective non-inva- ma-specific exosome RNAs in peripheral blood, sive “liquid biopsy” of lung adenocarcinomas. while assessing the feasibility and efficiency of this recently developed deep-sequencing tech- Key Words: nology for transcriptome profiling. Lung adenocarcinoma, Pseudogenes, Exosome, PATIENTS AND METHODS: Plasma-derived Biomarkers. exosome RNAs were isolated from 13 lung ad- enocarcinoma patients, 3 patients with benign lung diseases, and 15 healthy volunteers. RNA- seq analysis of ribosomal RNA-depleted total RNA was performed. RNAs differentially ex- Introduction pressed between lung adenocarcinoma and be- nign lung diseases or healthy volunteers were Lung cancer is the predominant cause of identified, followed by GO and KEGG pathway cancer-related death worldwide1. -

A Brain-Derived Neurotrophic Factor
www.nature.com/scientificreports OPEN A Brain-Derived Neurotrophic Factor Mimetic Is Sufcient to Restore Cone Photoreceptor Visual Received: 17 January 2017 Accepted: 25 August 2017 Function in an Inherited Blindness Published: xx xx xxxx Model Conor Daly1, Lisa Shine1, Theresa Hefernan1, Sudhakar Deeti1, Alison L. Reynolds 1, John J. O’Connor1, Eugène T. Dillon 1,2, David J. Dufy 3,5, Walter Kolch 1,3,4, Gerard Cagney1 & Breandán N. Kennedy 1 Controversially, histone deacetylase inhibitors (HDACi) are in clinical trial for the treatment of inherited retinal degeneration. Utilizing the zebrafsh dyeucd6 model, we determined if treatment with HDACi can rescue cone photoreceptor-mediated visual function. dye exhibit defective visual behaviour and retinal morphology including ciliary marginal zone (CMZ) cell death and decreased photoreceptor outer segment (OS) length, as well as gross morphological defects including hypopigmentation and pericardial oedema. HDACi treatment of dye results in signifcantly improved optokinetic (OKR) (~43 fold, p < 0.001) and visualmotor (VMR) (~3 fold, p < 0.05) responses. HDACi treatment rescued gross morphological defects and reduced CMZ cell death by 80%. Proteomic analysis of dye eye extracts suggested BDNF-TrkB and Akt signaling as mediators of HDACi rescue in our dataset. Co- treatment with the TrkB antagonist ANA-12 blocked HDACi rescue of visual function and associated Akt phosphorylation. Notably, sole treatment with a BDNF mimetic, 7,8-dihydroxyfavone hydrate, signifcantly rescued dye visual function (~58 fold increase in OKR, p < 0.001, ~3 fold increase in VMR, p < 0.05). In summary, HDACi and a BDNF mimetic are sufcient to rescue retinal cell death and visual function in a vertebrate model of inherited blindness. -
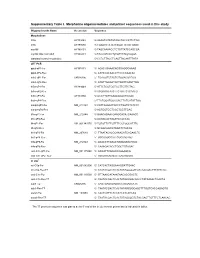
Supplementary Table I. Morpholino Oligonucleotides and Primer Sequences Used in This Study
Supplementary Table I. Morpholino oligonucleotides and primer sequences used in this study Oligonucleotide Name Accession Sequence Morpholinos tlr5a AY389449 5'-AAAGTGTATGTAGCTGCCATTCTGG tlr5b AY389450 5'-TGAATGTATATCCCATTCTGTGAGC myd88 AY388401 5'-TAGCAAAACCTCTGTTATCCAGCGA myd88 5bp mismatch AY388401 5'-TAcCAtAACCTgTGTTATCgAGgGA standard control morpholino 5'-CCTCTTACCTCAGTTACAATTTATA qRT-PCR ppial-qP1-Fw AY391451 5’- ACACTGAAACACGGAGGCAAAG ppial-qP2-Rev 5’- CATCCACAACCTTCCCGAACAC irak3-qP1-Fw CK026195 5’- TGAGGTCTACTGTGGACGATGG irak3-qP2-Rev 5’- ATGTTAGGATGCTGGTTGAGTTGG tlr5a-qP1-Fw AY389449 5’-ATTCTGGTGGTGCTTGTTGTAG tlr5a-qP2-Rev 5’-ACGAGGTAACTTCTGTTCTCAATG tlr5b-qP3-Fw AY389450 5’-GCGTTGTTGAAGAGGCTGGAC tlr5b-qP4-Rev 5’-TTCTGGATGGCCACTTCTCATATTGG mmp9-qP3-Fw NM_213123 5’-CATTAAAGATGCCCTGATGTATCCC mmp9-qP4-Rev 5’-AGTGGTGGTCCGTGGTTGAG il1b-qP1-Fw NM_212844 5’-GAACAGAATGAAGCACATCAAACC il1b-qP2-Rev 5’-ACGGCACTGAATCCACCAC il8-qP1-Fw XM_001342570 5’-TGTGTTATTGTTTTCCTGGCATTTC il8-qP2-Rev 5’-GCGACAGCGTGGATCTACAG ifn1-qP3-Fw NM_207640 5’- TTAATACACGCAAAGATGAGAACTC ifn1-qP4-Rev 5’- GCCAAGCCATTCGCAAGTAG tnfa-qP5-Fw NM_212829 5’- AGACCTTAGACTGGAGAGATGAC tnfa-qP6-Rev 5’- CAAAGACACCTGGCTGTAGAC cxcl-C1c-qP1-Fw NM_001115060 5’- GGCATTCACACCCAAAGCG cxcl-C1c-qP2_Rev 5’- GCGAGCACGATTCACGAGAG * In situ ccl-C5a-Fw NM_001082906 5’- CATCACTAGGAAAGGATTGAAC ccl-C5a-Rev-T7 5’- TAATACGACTCACTATAGGGGATGTCAAAGACTTTATTCAC cxcl-C1c-Fw NM_001115060 5’- GTTAAACATAAATAACACCGACTC cxcl-C1c-Rev-T7 5’- TAATACGACTCACTATAGGGACACCCTATAAAACTGAGTA irak3-Fw CK026195 5’- CAGTGAGAGAGGCATGAAACATC