Autophagy Supports Color Vision
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Browsing Genes and Genomes with Ensembl
The Bioinformatics Roadshow Tórshavn, The Faroe Islands 28-29 November 2012 BROWSING GENES AND GENOMES WITH ENSEMBL EXERCISES AND ANSWERS 1 BROWSER 3 BIOMART 8 VARIATION 13 COMPARATIVE GENOMICS 18 2 Note: These exercises are based on Ensembl version 69 (October 2012). After in future a new version has gone live, version 69 will still be available at http://e69.ensembl.org/. If your answer doesn’t correspond with the given answer, please consult the instructor. ______________________________________________________________ BROWSER ______________________________________________________________ Exercise 1 – Exploring a gene (a) Find the human F9 (coagulation factor IX) gene. On which chromosome and which strand of the genome is this gene located? How many transcripts (splice variants) have been annotated for it? (b) What is the longest transcript? How long is the protein it encodes? Has this transcript been annotated automatically (by Ensembl) or manually (by Havana)? How many exons does it have? Are any of the exons completely or partially untranslated? (c) Have a look at the external references for ENST00000218099. What is the function of F9? (d) Is it possible to monitor expression of ENST00000218099 with the ILLUMINA HumanWG_6_V2 microarray? If so, can it also be used to monitor expression of the other two transcripts? (e) In which part (i.e. the N-terminal or C-terminal half) of the protein encoded by ENST00000218099 does its peptidase activity reside? (f) Have any missense variants been discovered for the protein encoded by ENST00000218099? (g) Is there a mouse orthologue predicted for the human F9 gene? (h) If you have yourself a gene of interest, explore what information Ensembl displays about it! ______________________________________________________________ Answer (a) 8 Go to the Ensembl homepage (http://www.ensembl.org/). -

Structure of Cone Photoreceptors
Progress in Retinal and Eye Research 28 (2009) 289–302 Contents lists available at ScienceDirect Progress in Retinal and Eye Research journal homepage: www.elsevier.com/locate/prer Structure of cone photoreceptors Debarshi Mustafi a, Andreas H. Engel a,b, Krzysztof Palczewski a,* a Department of Pharmacology, Case Western Reserve University, Cleveland, OH 44106-4965, USA b Center for Cellular Imaging and Nanoanalytics, M.E. Mu¨ller Institute, Biozentrum, WRO-1058, Mattenstrasse 26, CH 4058 Basel, Switzerland abstract Keywords: Although outnumbered more than 20:1 by rod photoreceptors, cone cells in the human retina mediate Cone photoreceptors daylight vision and are critical for visual acuity and color discrimination. A variety of human diseases are Rod photoreceptors characterized by a progressive loss of cone photoreceptors but the low abundance of cones and the Retinoids absence of a macula in non-primate mammalian retinas have made it difficult to investigate cones Retinoid cycle directly. Conventional rodents (laboratory mice and rats) are nocturnal rod-dominated species with few Chromophore Opsins cones in the retina, and studying other animals with cone-rich retinas presents various logistic and Retina technical difficulties. Originating in the early 1900s, past research has begun to provide insights into cone Vision ultrastructure but has yet to afford an overall perspective of cone cell organization. This review Rhodopsin summarizes our past progress and focuses on the recent introduction of special mammalian models Cone pigments (transgenic mice and diurnal rats rich in cones) that together with new investigative techniques such as Enhanced S-cone syndrome atomic force microscopy and cryo-electron tomography promise to reveal a more unified concept of cone Retinitis pigmentosa photoreceptor organization and its role in retinal diseases. -

How Photons Start Vision DENIS BAYLOR Department of Neurobiology, Sherman Fairchild Science Building, Stanford University School of Medicine, Stanford, CA 94305
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 560-565, January 1996 Colloquium Paper This paper was presented at a coUoquium entitled "Vision: From Photon to Perception," organized by John Dowling, Lubert Stryer (chair), and Torsten Wiesel, held May 20-22, 1995, at the National Academy of Sciences in Irvine, CA. How photons start vision DENIS BAYLOR Department of Neurobiology, Sherman Fairchild Science Building, Stanford University School of Medicine, Stanford, CA 94305 ABSTRACT Recent studies have elucidated how the ab- bipolar and horizontal cells. Light absorbed in the pigment acts sorption of a photon in a rod or cone cell leads to the to close cationic channels in the outer segment, causing the generation of the amplified neural signal that is transmitted surface membrane of the entire cell to hyperpolarize. The to higher-order visual neurons. Photoexcited visual pigment hyperpolarization relays visual information to the synaptic activates the GTP-binding protein transducin, which in turn terminal, where it slows ongoing transmitter release. The stimulates cGMP phosphodiesterase. This enzyme hydrolyzes cationic channels in the outer segment are controlled by the cGMP, allowing cGMP-gated cationic channels in the surface diffusible cytoplasmic ligand cGMP, which binds to channels membrane to close, hyperpolarize the cell, and modulate in darkness to hold them open. Light closes channels by transmitter release at the synaptic terminal. The kinetics of lowering the cytoplasmic concentration of cGMP. The steps reactions in the cGMP cascade limit the temporal resolution that link light absorption to channel closure in a rod are of the visual system as a whole, while statistical fluctuations illustrated schematically in Fig. -

Differentiation of Human Embryonic Stem Cells Into Cone Photoreceptors
© 2015. Published by The Company of Biologists Ltd | Development (2015) 142, 3294-3306 doi:10.1242/dev.125385 RESEARCH ARTICLE STEM CELLS AND REGENERATION Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFβ and Wnt signaling Shufeng Zhou1,*, Anthony Flamier1,*, Mohamed Abdouh1, Nicolas Tétreault1, Andrea Barabino1, Shashi Wadhwa2 and Gilbert Bernier1,3,4,‡ ABSTRACT replacement therapy may stop disease progression or restore visual Cone photoreceptors are required for color discrimination and high- function. However, a reliable and abundant source of human cone resolution central vision and are lost in macular degenerations, cone photoreceptors is not currently available. This limitation may be and cone/rod dystrophies. Cone transplantation could represent a overcome using embryonic stem cells (ESCs). ESCs originate from therapeutic solution. However, an abundant source of human cones the inner cell mass of the blastocyst and represent the most primitive remains difficult to obtain. Work performed in model organisms stem cells. Human ESCs (hESCs) can develop into cells and tissues suggests that anterior neural cell fate is induced ‘by default’ if BMP, of the three primary germ layers and be expanded indefinitely TGFβ and Wnt activities are blocked, and that photoreceptor genesis (Reubinoff et al., 2000; Thomson et al., 1998). operates through an S-cone default pathway. We report here that Work performed in amphibians and chick suggests that primordial Coco (Dand5), a member of the Cerberus gene family, is expressed cells adopt a neural fate in the absence of alternative cues (Muñoz- in the developing and adult mouse retina. Upon exposure Sanjuán and Brivanlou, 2002). -

Antioxidants Reduce Cone Cell Death in a Model of Retinitis Pigmentosa
Antioxidants reduce cone cell death in a model of retinitis pigmentosa Keiichi Komeima, Brian S. Rogers, Lili Lu, and Peter A. Campochiaro* Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 21287-9277 Edited by Jeremy Nathans, Johns Hopkins University School of Medicine, and approved June 9, 2006 (received for review May 16, 2006) Retinitis pigmentosa (RP) is a label for a group of diseases caused to release a chemoattractant for RPE cells stimulating their tran- by a large number of mutations that result in rod photoreceptor sretinal migration. It is remarkable that the death of rods can have cell death followed by gradual death of cones. The mechanism of these remote effects long after they have departed. cone cell death is uncertain. Rods are a major source of oxygen The loss of rods has delayed effects on other cells in addition to utilization in the retina and, after rods die, the level of oxygen in vascular and RPE cells. After the death of rods, cone photorecep- the outer retina is increased. In this study, we used the rd1 mouse tors begin to die. Depletion of rods is responsible for night model of RP to test the hypothesis that cones die from oxidative blindness, the first symptom of RP, but it is the death of cones that damage. A mixture of antioxidants was selected to try to maximize is responsible for the gradual constriction of the visual fields and protection against oxidative damage achievable by exogenous eventual blindness. The mechanism of the gradual death of cones supplements; ␣-tocopherol (200 mg͞kg), ascorbic acid (250 mg͞kg), is one of the key unsolved mysteries of RP. -

Viewpoints, Specific- Ally the Relative Growth of the Eye, Directional Sensitivity of Ommatidia, and Sensitivity to Polarized Light
N. SHUSTER.~JR..PH.D.. Zeitschrift fur Zellforschung 87, 278-291 (1968) The Morphology of the Eyes of Limulus I. Cornea and Epidermis of the Compound Eye * WOLFH. FAHRENBACH OregonRegional Primate ResearchCenter, Beaverton, Oregon (U.S.A. ReceivedDecember 11, 1967 * This study constitutes publication No. 288 from the Oregon Regional Primate Research Center, supported in part by Grants FR 00163 and NBO77l7-01 from the National Institutes of Health and in part by a Bob Hope Fight For Sight Grant-in-Aid of the National Council to Combat Blindness, Inc. The author wishes to thank Mrs. AUDREY GRIFFIN for patient and excellent technical assistance. '.. c. Summary. The dioptric apparatus of the Limulu8 compound eye is composed of the corneal cuticle with its internally projecting cuticular cones and the specialized underlying epidermis. The latter is composed of three distinct cell types. The guanophores, located between cuticular cones, contain guanine as a reflecting pigment. The distal pigment cells, which clothe the sides of the cuticular cones and form a sheath around the underlying ommatidium, contain massive bundles of microtubules, abundant pigment droplets and a large Golgi system. The cone cells are positioned between the flattened tip of the cuticular cone and the apex of the ommatidium. They serve to anchor the retinula cells to the cuticle and, by virtue of long processesalong the periphery of the rhabdome, perform a glial function with respect to the interaction of adjacent retinula cells. The geometry and fine structure of the dioptric apparatus provide supporting evidence for the wide angle of acceptance and lack of polarized light perception by the ommatidia. -

Shedding New Light on the Generation of the Visual Chromophore PERSPECTIVE Krzysztof Palczewskia,B,C,1 and Philip D
PERSPECTIVE Shedding new light on the generation of the visual chromophore PERSPECTIVE Krzysztof Palczewskia,b,c,1 and Philip D. Kiserb,d Edited by Jeremy Nathans, Johns Hopkins University School of Medicine, Baltimore, MD, and approved July 9, 2020 (received for review May 16, 2020) The visual phototransduction cascade begins with a cis–trans photoisomerization of a retinylidene chro- mophore associated with the visual pigments of rod and cone photoreceptors. Visual opsins release their all-trans-retinal chromophore following photoactivation, which necessitates the existence of pathways that produce 11-cis-retinal for continued formation of visual pigments and sustained vision. Proteins in the retinal pigment epithelium (RPE), a cell layer adjacent to the photoreceptor outer segments, form the well- established “dark” regeneration pathway known as the classical visual cycle. This pathway is sufficient to maintain continuous rod function and support cone photoreceptors as well although its throughput has to be augmented by additional mechanism(s) to maintain pigment levels in the face of high rates of photon capture. Recent studies indicate that the classical visual cycle works together with light-dependent pro- cesses in both the RPE and neural retina to ensure adequate 11-cis-retinal production under natural illu- minances that can span ten orders of magnitude. Further elucidation of the interplay between these complementary systems is fundamental to understanding how cone-mediated vision is sustained in vivo. Here, we describe recent -
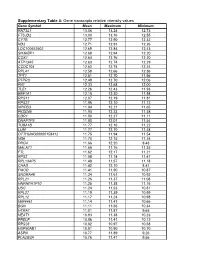
Gene Transcripts Relative Intensity Values
Supplementary Table 4: Gene transcripts relative intensity values Gene Symbol Mean Maximum Minimum RN7SL1 13.06 13.24 12.73 FTSJD2 13.00 13.16 12.55 CYTB 12.77 12.90 12.32 ND2 12.71 12.91 12.26 LOC100652902 12.69 12.84 12.43 SH3KBP1 12.68 12.94 12.20 COX1 12.64 12.76 12.20 ATP13A5 12.63 12.74 12.29 CCDC104 12.62 12.74 12.24 RPL41 12.58 12.66 12.36 TPT1 12.51 12.70 11.96 PTPRO 12.49 12.70 12.06 FN1 12.33 12.68 12.00 TLE1 12.26 12.43 11.93 EEF1A1 12.15 12.30 11.98 RPS11 12.07 12.19 11.81 RPS27 11.96 12.10 11.72 NPIPB3 11.94 12.21 11.65 FKSG49 11.94 12.23 11.58 CDR1 11.90 12.27 11.11 DNAPTP3 11.80 12.07 11.54 TUBA1B 11.77 12.16 11.22 LUM 11.77 12.10 11.48 OTTHUMG00000158412 11.75 11.94 11.54 ND6 11.70 12.15 11.24 PRG4 11.66 12.20 9.43 MALAT1 11.65 11.76 11.35 FTL 11.62 12.17 11.21 RPS2 11.58 11.74 11.47 RPL13AP5 11.48 11.57 11.18 CHAD 11.42 12.10 8.41 FMOD 11.41 11.90 10.87 SNORA48 11.34 11.61 10.92 RPL21 11.26 11.37 11.08 HNRNPA1P10 11.25 11.38 11.16 UBC 11.24 11.53 10.81 RPL27 11.19 11.39 10.99 RPL12 11.17 11.24 10.98 MIR4461 11.14 11.47 10.66 BGN 11.11 11.66 10.34 HTRA1 11.01 11.87 9.65 NEAT1 10.93 11.38 10.23 PRELP 10.86 11.41 10.12 RPS28 10.82 10.97 10.58 HSP90AB1 10.81 10.90 10.70 ASPN 10.77 11.89 9.26 PLA2G2A 10.76 11.47 9.56 H3F3A 10.69 10.86 10.41 EEF1G 10.69 10.94 10.11 C6orf48 10.64 11.02 10.35 UBA52 10.58 10.75 10.08 CTGF 10.57 11.33 9.66 MGP 10.57 11.22 10.09 YBX1 10.56 10.79 10.01 MT1X 10.56 11.89 9.88 NPC2 10.55 10.80 10.12 LAPTM4A 10.51 10.78 10.18 ITM2B 10.51 10.68 10.34 IBSP 10.50 11.50 7.17 NPIPB5 10.50 10.73 10.26 TUBA1A -

This Item Is the Archived Peer-Reviewed Author-Version Of
This item is the archived peer-reviewed author-version of: Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Reference: Houbrechts Anne M., Vergauwen Lucia, Bagci Enise, Van houcke Jolien, Heijlen Marjolein, Kulemeka Bernard, Hyde David R., Knapen Dries, Darras Veerle M..- Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Molecular and cellular endocrinology - ISSN 0303-7207 - 424(2016), p. 81-93 Full text (Publishers DOI): http://dx.doi.org/doi:10.1016/j.mce.2016.01.018 To cite this reference: http://hdl.handle.net/10067/1314550151162165141 Institutional repository IRUA 1 Deiodinase knockdown affects zebrafish eye development at the level 2 of gene expression, morphology and function. 3 Anne M. Houbrechts1*, Lucia Vergauwen2*, Enise Bagci2,3, Jolien Van houcke1, Marjolein 4 Heijlen1, Bernard Kulemeka4, David R. Hyde4, Dries Knapen2, Veerle M. Darras1 5 1 Laboratory of Comparative Endocrinology, Department of Biology, Division of 6 Animal Physiology and Neurobiology, KU Leuven, 3000 Leuven, Belgium 7 2 Zebrafishlab, Veterinary Physiology and Biochemistry, Department of Veterinary 8 Sciences, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 9 3 Systemic Physiological & Ecotoxicological Research, Department of Biology, 10 University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 11 4 Department of Biological Sciences and Center for Zebrafish Research, University of 12 Notre Dame, Notre Dame, -

A Brain-Derived Neurotrophic Factor
www.nature.com/scientificreports OPEN A Brain-Derived Neurotrophic Factor Mimetic Is Sufcient to Restore Cone Photoreceptor Visual Received: 17 January 2017 Accepted: 25 August 2017 Function in an Inherited Blindness Published: xx xx xxxx Model Conor Daly1, Lisa Shine1, Theresa Hefernan1, Sudhakar Deeti1, Alison L. Reynolds 1, John J. O’Connor1, Eugène T. Dillon 1,2, David J. Dufy 3,5, Walter Kolch 1,3,4, Gerard Cagney1 & Breandán N. Kennedy 1 Controversially, histone deacetylase inhibitors (HDACi) are in clinical trial for the treatment of inherited retinal degeneration. Utilizing the zebrafsh dyeucd6 model, we determined if treatment with HDACi can rescue cone photoreceptor-mediated visual function. dye exhibit defective visual behaviour and retinal morphology including ciliary marginal zone (CMZ) cell death and decreased photoreceptor outer segment (OS) length, as well as gross morphological defects including hypopigmentation and pericardial oedema. HDACi treatment of dye results in signifcantly improved optokinetic (OKR) (~43 fold, p < 0.001) and visualmotor (VMR) (~3 fold, p < 0.05) responses. HDACi treatment rescued gross morphological defects and reduced CMZ cell death by 80%. Proteomic analysis of dye eye extracts suggested BDNF-TrkB and Akt signaling as mediators of HDACi rescue in our dataset. Co- treatment with the TrkB antagonist ANA-12 blocked HDACi rescue of visual function and associated Akt phosphorylation. Notably, sole treatment with a BDNF mimetic, 7,8-dihydroxyfavone hydrate, signifcantly rescued dye visual function (~58 fold increase in OKR, p < 0.001, ~3 fold increase in VMR, p < 0.05). In summary, HDACi and a BDNF mimetic are sufcient to rescue retinal cell death and visual function in a vertebrate model of inherited blindness. -
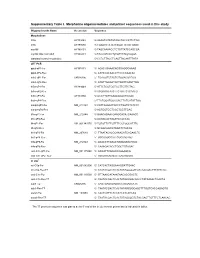
Supplementary Table I. Morpholino Oligonucleotides and Primer Sequences Used in This Study
Supplementary Table I. Morpholino oligonucleotides and primer sequences used in this study Oligonucleotide Name Accession Sequence Morpholinos tlr5a AY389449 5'-AAAGTGTATGTAGCTGCCATTCTGG tlr5b AY389450 5'-TGAATGTATATCCCATTCTGTGAGC myd88 AY388401 5'-TAGCAAAACCTCTGTTATCCAGCGA myd88 5bp mismatch AY388401 5'-TAcCAtAACCTgTGTTATCgAGgGA standard control morpholino 5'-CCTCTTACCTCAGTTACAATTTATA qRT-PCR ppial-qP1-Fw AY391451 5’- ACACTGAAACACGGAGGCAAAG ppial-qP2-Rev 5’- CATCCACAACCTTCCCGAACAC irak3-qP1-Fw CK026195 5’- TGAGGTCTACTGTGGACGATGG irak3-qP2-Rev 5’- ATGTTAGGATGCTGGTTGAGTTGG tlr5a-qP1-Fw AY389449 5’-ATTCTGGTGGTGCTTGTTGTAG tlr5a-qP2-Rev 5’-ACGAGGTAACTTCTGTTCTCAATG tlr5b-qP3-Fw AY389450 5’-GCGTTGTTGAAGAGGCTGGAC tlr5b-qP4-Rev 5’-TTCTGGATGGCCACTTCTCATATTGG mmp9-qP3-Fw NM_213123 5’-CATTAAAGATGCCCTGATGTATCCC mmp9-qP4-Rev 5’-AGTGGTGGTCCGTGGTTGAG il1b-qP1-Fw NM_212844 5’-GAACAGAATGAAGCACATCAAACC il1b-qP2-Rev 5’-ACGGCACTGAATCCACCAC il8-qP1-Fw XM_001342570 5’-TGTGTTATTGTTTTCCTGGCATTTC il8-qP2-Rev 5’-GCGACAGCGTGGATCTACAG ifn1-qP3-Fw NM_207640 5’- TTAATACACGCAAAGATGAGAACTC ifn1-qP4-Rev 5’- GCCAAGCCATTCGCAAGTAG tnfa-qP5-Fw NM_212829 5’- AGACCTTAGACTGGAGAGATGAC tnfa-qP6-Rev 5’- CAAAGACACCTGGCTGTAGAC cxcl-C1c-qP1-Fw NM_001115060 5’- GGCATTCACACCCAAAGCG cxcl-C1c-qP2_Rev 5’- GCGAGCACGATTCACGAGAG * In situ ccl-C5a-Fw NM_001082906 5’- CATCACTAGGAAAGGATTGAAC ccl-C5a-Rev-T7 5’- TAATACGACTCACTATAGGGGATGTCAAAGACTTTATTCAC cxcl-C1c-Fw NM_001115060 5’- GTTAAACATAAATAACACCGACTC cxcl-C1c-Rev-T7 5’- TAATACGACTCACTATAGGGACACCCTATAAAACTGAGTA irak3-Fw CK026195 5’- CAGTGAGAGAGGCATGAAACATC -

The Topography of Rods, Cones and Intrinsically
RESEARCH ARTICLE The topography of rods, cones and intrinsically photosensitive retinal ganglion cells in the retinas of a nocturnal (Micaelamys namaquensis) and a diurnal (Rhabdomys pumilio) rodent Ingrid van der Merwe1☯*, A kos LukaÂts2☯, Veronika BlaÂhovaÂ3, Maria K. Oosthuizen1, Nigel a1111111111 C. Bennett1, Pavel Němec3* a1111111111 a1111111111 1 Mammal Research Institute, Department of Zoology and Entomology, University of Pretoria, Pretoria, South Africa, 2 Department of Anatomy, Histology and Embryology, Semmelweis University, Budapest, a1111111111 Hungary, 3 Department of Zoology, Charles University, Prague, Czech Republic a1111111111 ☯ These authors contributed equally to this work. * [email protected] (IVDM); [email protected] (PN) OPEN ACCESS Abstract Citation: van der Merwe I, LukaÂts AÂ, BlaÂhova V, Oosthuizen MK, Bennett NC, Němec P (2018) The We used immunocytochemistry to determine the presence and topographical density distri- topography of rods, cones and intrinsically butions of rods, cones, and intrinsically photosensitive retinal ganglion cells (ipRGCs) in the photosensitive retinal ganglion cells in the retinas four-striped field mouse (Rhabdomys pumilio) and the Namaqua rock mouse (Micaelamys of a nocturnal (Micaelamys namaquensis) and a diurnal (Rhabdomys pumilio) rodent. PLoS ONE 13 namaquensis). Both species possessed duplex retinas that were rod dominated. In R. pumi- (8): e0202106. https://doi.org/10.1371/journal. lio, the density of both cones and rods were high (cone to rod ratio: 1:1.23) and reflected the pone.0202106 species' fundamentally diurnal, but largely crepuscular lifestyle. Similarly, the ratio of cones Editor: Gianluca Tosini, Morehouse School of to rods in M. namaquensis (1:12.4) reflected its nocturnal lifestyle. Similar rod density peaks Medicine, UNITED STATES were observed (R.