22.4 Aldol Addition and Aldol Condensation 1063
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jean-Baptiste Charles Joseph Bélanger (1790-1874), the Backwater Equation and the Bélanger Equation
THE UNIVERSITY OF QUEENSLAND DIVISION OF CIVIL ENGINEERING REPORT CH69/08 JEAN-BAPTISTE CHARLES JOSEPH BÉLANGER (1790-1874), THE BACKWATER EQUATION AND THE BÉLANGER EQUATION AUTHOR: Hubert CHANSON HYDRAULIC MODEL REPORTS This report is published by the Division of Civil Engineering at the University of Queensland. Lists of recently-published titles of this series and of other publications are provided at the end of this report. Requests for copies of any of these documents should be addressed to the Civil Engineering Secretary. The interpretation and opinions expressed herein are solely those of the author(s). Considerable care has been taken to ensure accuracy of the material presented. Nevertheless, responsibility for the use of this material rests with the user. Division of Civil Engineering The University of Queensland Brisbane QLD 4072 AUSTRALIA Telephone: (61 7) 3365 3619 Fax: (61 7) 3365 4599 URL: http://www.eng.uq.edu.au/civil/ First published in 2008 by Division of Civil Engineering The University of Queensland, Brisbane QLD 4072, Australia © Chanson This book is copyright ISBN No. 9781864999211 The University of Queensland, St Lucia QLD JEAN-BAPTISTE CHARLES JOSEPH BÉLANGER (1790-1874), THE BACKWATER EQUATION AND THE BÉLANGER EQUATION by Hubert CHANSON Professor, Division of Civil Engineering, School of Engineering, The University of Queensland, Brisbane QLD 4072, Australia Ph.: (61 7) 3365 3619, Fax: (61 7) 3365 4599, Email: [email protected] Url: http://www.uq.edu.au/~e2hchans/ REPORT No. CH69/08 ISBN 9781864999211 Division of Civil Engineering, The University of Queensland August 2008 Jean-Baptiste BÉLANGER (1790-1874) (Courtesy of the Bibliothèque de l'Ecole Nationale Supérieure des Ponts et Chaussées) Abstract In an open channel, the transition from a high-velocity open channel flow to a fluvial motion is a flow singularity called a hydraulic jump. -
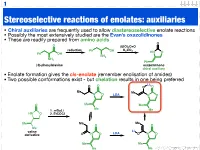
Stereoselective Reactions of Enolates: Auxiliaries
1 Stereoselective reactions of enolates: auxiliaries • Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions • Possibly the most extensively studied are the Evan’s oxazolidinones • These are readily prepared from amino acids O O (EtO)2C=O reduction K CO Ph OH 2 3 HN O Ph OH NH2 NH2 Ph (S)-phenylalanine oxazolidinone chiral auxiliary • Enolate formation gives the cis-enolate (remember enolisation of amides) • Two possible conformations exist - but chelation results in one being preferred Li O O O O Me Me N O LDA N O O Me Me Me 1. n-BuLi Me HN O 2. EtCOCl Me Me Me O O Me Li valine LDA derivative O N O O N O Me Me Me Me 123.702 Organic Chemistry 2 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 3 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 4 Diastereoselective functionalisation Li O O O O Br LDA Me Me N O N O H Ph Ph 96% de H O -
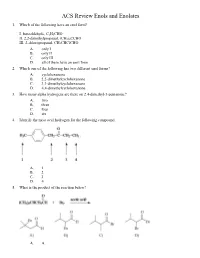
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Diversity-Oriented Combinatorial Biosynthesis of Benzenediol Lactone Scaffolds by Subunit Shuffling of Fungal Polyketide Synthases
Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthases Yuquan Xua,b,1, Tong Zhouc,1, Shuwei Zhangc, Patricia Espinosa-Artilesb, Luoyi Wangc, Wei Zhanga, Min Lina, A. A. Leslie Gunatilakab,d, Jixun Zhanc,2, and István Molnárb,d,2 aBiotechnology Research Institute, The Chinese Academy of Agricultural Sciences, Beijing 100081, P. R. China; bNatural Products Center, School of Natural Resources and the Environment, University of Arizona, Tucson, AZ 85706; cDepartment of Biological Engineering, Utah State University, Logan, UT 84322; and dBio5 Institute, University of Arizona, Tucson, AZ 85721 Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved June 23, 2014 (received for review April 16, 2014) Combinatorial biosynthesis aspires to exploit the promiscuity of biosynthesis inhibitory activities in animals. 10,11-dehydrocurvularin microbial anabolic pathways to engineer the synthesis of new (7;Fig.1)isaDALwitha12-memberedring(DAL12)that chemical entities. Fungal benzenediol lactone (BDL) polyketides modulates the heat shock response and the immune system (8, 9). are important pharmacophores with wide-ranging bioactivities, BDL scaffolds are biosynthesized by pairs of collaborating, including heat shock response and immune system modulatory sequentially acting iterative polyketide synthases (iPKSs) (3) – effects. Their biosynthesis on a pair of sequentially acting iterative forming quasi-modular BDL synthases (BDLSs) (Fig. 1) (11 14). polyketide synthases (iPKSs) offers a test case for the modulariza- Each of the BDLS subunits catalyze recursive, decarboxylative tion of secondary metabolic pathways into “build–couple–pair” Claisen condensations of malonyl-CoA using a single core set of ketoacyl synthase (KS), acyl transferase (AT), and acyl carrier combinatorial synthetic schemes. -

Experiment 19 — Aldol Condensation
Chem 22 Spring 2010 Experiment 19 — Aldol Condensation _____________________________________________________________________________ Pre-lab preparation. (1) Write the mechanism of the base-catalyzed aldol condensation of acetone and a generalized aromatic aldehyde, Ar–CH=O to give the α,β-unsaturated product (i.e. the reaction at the top of the next page, but with a 1:1 ratio of ketone and aldehyde). Remember that dehydration in this case occurs under basic conditions, so it can't start with protonation of the hydroxyl group. Nor can it go via an E2 pathway. (2) Draw the structures of all the possible aldehyde and ketone reactants (not the 25 possible condensation products!). (3) The new CC double bonds of the condensation products are E rather than Z, as shown in the acetone example. Why? (4) What's the purpose of rinsing the crude product with dilute acetic acid, followed by ethanol? (5) What is the procedure for carrying out a single-solvent recrystallization? Write this out in detail. The aldol condensation has historically been one of the favorite tools in the synthetic organic chemist's repertoire because of its versatility in forming new CC bonds. Since its discovery in the 1870s the aldol condensation has been use extensively in the synthesis of natural products and other complex molecules. In a typical base-catalyzed aldol condensation an enolate ion attacks the carbonyl group of an aldehyde or ketone. This carbonyl addition produces a β-hydroxy carbonyl compound. In many cases the initially formed condensation product undergoes an "E1cB" dehydration to produce an α,β-unsaturated carbonyl compound as the final product. -

Page 1 of 108 RSC Advances
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 108 RSC Advances Applications of oxazolidinones as chiral auxiliaries in the asymmetric alkylation reaction applied to total synthesis Majid M. Heravi,* Vahideh Zadsirjan, Behnaz Farajpour Department of Chemistry, School of Science, Alzahra University, Vanak, Tehran, Iran Email: [email protected] Abstract Various chiral oxazolidinones (Evans' oxazolidinones) have been employed as effective chiral auxiliaries in the asymmetric alkylation of different enolates. This strategy has been found promising and successful when used as key step (steps) in the total synthesis of several biologically active natural products. In this report, we try to underscore the applications of Manuscript oxazolidinones as chiral auxiliary in asymmetric alkylation, and particularly in crucial chiral inducing steps in the total synthesis of natural products, showing biological activities. -

Aldol Condensation- Aldehyde (Or Ketone) Enolate Condenses with Aldehyde (Or Ketone)
Chem 232 Summary of Alpha Substitutions page 1 Aldol Condensation- aldehyde (or ketone) enolate condenses with aldehyde (or ketone): O CH O O H 3 H CH 3 CH3 C C C C CH C C CH2 CH2 H -H O H H O OH 2 H nucleophile electrophile -hydroxy aldehyde -unsaturated aldehyde The nucleophile can be a ketone enolate or aldehyde enolate and the electrophile (shaded) can be an aldehyde or ketone. Crossed Aldol- Condensation between two different carbonyls. The component without hydrogens is the electrophile: O O O OH O C CH C C CH CH3 C H CH -H2O CH 2 CH3 H 3 ketone enolate no -hydrogens -unsaturated ketone Aldol Cyclizations- A dicarbonyl produces an enolate and carbonyl in the same molecule: enolate from a O OH 1,5-diketone CH OH 3 CH3 O O -H2O O CH2 CH3 CH3 CH2 O Claisen Condensation- Similar to Aldol condensation except the nucleophile is an ester enolate; O O O O O O + EtO C CH C OEt EtO C CH2 C EtO C CH2 CH3 C OEt 2 ketoester CH3 CH3 Dieckmann Cyclization- Internal Claisen condensation similar to Aldol cyclization. A 1,6 diester gives a 5-membered ring and a 1,7 diester gives a 6-membered ring: O OEt O OEt cyclic ketoester C C OEt O O Crossed Claisen- Similar to crossed Aldol- Electrophile has nohydrogens: O O O O C C EtO EtO C CH2 EtO C CH2 ketoester Variations on Crossed Claisen- ketone enolate and ester condensation. Esters, carbonates, formates and oxalates are common electrophiles: O O O O O O O O H C OEt H EtO C OEt OEt ethyl formate -ketoaldehyde diethyl carbonate -ketoester O O O O O OEt diketoester EtO C C OEt O diethyl oxalate -

Charles Fréderic Gerhardt Jaime Wisniak
PARA QUITARLE EL POLVO La química en la historia, para la enseñanza Charles Fréderic Gerhardt Jaime Wisniak Resumen Charles-Victor Lobstein (1809-1863). Samuel Ger- Charles Fréderic Gerhardt (1816-1856) fue uno de los hardt, born in Switzerland, came from a well-known químicos más importantes del siglo diecinueve, cu - family of brewers. At a young age he moved to yas investigaciones y teorías ejercieron una poderosa Strasbourg where he found employment in the influencia en el desarrollo de la química. Su teoría Turckheim bank and married. His skills led to a fast de los tipos fue significativa en impulsar hacia ade- career and thus to provide his family with a prosper- lante la clasificación orgánica, poniéndola en una ous and cultured home. forma más racional, y en destronar el enfoque dua - Between 1824 and 1831 Charles attended the lista. La teoría de los tipos evolucionó hacia la idea local Gymnase Protestant, an institution controlled by de valencia. Gerhardt generalizó el concepto de the Lutheran Augsburg Confession. According to homología y equivalentes. Fue asimismo responsa- Carneiro (1993) the Gymnasium had been founded ble de la síntesis de un gran número de compuestos in 1538 by Jean Sturm (1507-1589), a German Lu - orgánicos, entre ellos anhidridos y cloruros de ácido, theran reformed who advocated and practiced the derivados del ácido salicílico, anilidas, y fosfamidas. propagation of knowledge through teaching and publication. This institution was highly regarded in Abstract the educational circle; it had resisted several at- Charles Fréderic Gerhardt (1816-1856) was one of tempts by the Ministry of Instruction to integrate it the most important chemists of the nineteenth cen- into the official Lycée program, particularly after the tury and whose researches and theories exerted a anti clerical atmosphere that was prevalent after powerful influence in the development of chemistry. -

Redalyc.Joseph Achille Le Bel. His Life and Works
Revista CENIC. Ciencias Químicas ISSN: 1015-8553 [email protected] Centro Nacional de Investigaciones Científicas Cuba Wisniak, Jaime Joseph Achille Le Bel. His Life and Works Revista CENIC. Ciencias Químicas, vol. 33, núm. 1, enero-abril, 2002, pp. 35-43 Centro Nacional de Investigaciones Científicas La Habana, Cuba Available in: http://www.redalyc.org/articulo.oa?id=181625999008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista CENIC Ciencias Químicas, Vol. 33, No. 1, 2002. RESEÑA BIOGRAFICA Joseph Achille Le Bel. His Life and Works Jaime Wisniak Department of Chemical Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel 84105. [email protected]. Recibido: 26 de abril del 2001. Aceptado: 22 de mayo del 2001. Palabras clave: Le Bel, Química, estereoquímica, actividad óptica, cosmogonia Key words: Le Bel, Chemistry, stereoquímica, optical activity, cosmogony. RESUMEN. Joseph Achille Le Bel es un ejemplo de científicos como Réaumur The same year his father passed que investigaron muchÍsimos temas, pero solo son recordados por uno. Le Bel away and his two sisters, Marie and es un nombre bien conocido por los estudiantes de Química en general, y Emma, took charge of the family in- estereoquímica en particular. El nos dejo los principios básicos que determinan dustry and in this way allowed Le las condiciones geométricas que un compuesto de carbón debe satisfacer para Bel to continue chemical studies. -

Early Russian Organic Chemists and Their Legacy
SpringerBriefs in Molecular Science Early Russian Organic Chemists and Their Legacy Bearbeitet von David Lewis 1. Auflage 2012. Taschenbuch. xii, 136 S. Paperback ISBN 978 3 642 28218 8 Format (B x L): 15,5 x 23,5 cm Gewicht: 237 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Chemie Allgemein > Geschichte der Chemie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Chapter 2 Beginnings 2.1 Introduction At the start of the twentieth century, organic chemistry was not yet 75 years old as a separate and legitimate sub-discipline of the science. Considerable progress had been made in these first seven decades, and the stage was set for the dramatic advances in the science to come in the following century. Most practicing organic chemists are familiar with many of the great German, French and English organic chemists whose work helped the fledgling discipline grow, but few are familiar with the role that Russian organic chemists of the nineteenth and early twentieth century played in the development of the science. And this is in spite of the fact that many of the named rules and reactions that one studies in the first course in organic chemistry are, in fact, of Russian origin. It is the intent of this book to help rectify that deficiency. -

Aldol Reactions: E-Enolates and Anti-Selectivity
Utah State University DigitalCommons@USU All Graduate Plan B and other Reports Graduate Studies 5-2005 Aldol Reactions: E-Enolates and Anti-Selectivity Matthew Grant Anderson Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/gradreports Part of the Organic Chemistry Commons Recommended Citation Anderson, Matthew Grant, "Aldol Reactions: E-Enolates and Anti-Selectivity" (2005). All Graduate Plan B and other Reports. 1312. https://digitalcommons.usu.edu/gradreports/1312 This Report is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Plan B and other Reports by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. ALDOL REACTIONS: E-ENOLATES AND ANTI-SELECTIVITY Prepared By: MATTHEW GRANT ANDERSON A non-thesis paper submitted in partial fulfillment of the requirement for a Plan B Degree of Masters of Science in Organic Chemistry UTAH STATE UNIVERSITY Logan, Utah 2005 Contents Page CONTENTS ...................................................................................... .i LIST OF TABLES, FIGURES AND SCHEMES ....................................... ii,iii ABSTRACT .................................................................................... iv CHAPTER I. ALDOL REACTIONS:E-ENOLATES AND ANTI SELECTIVITY ......... 1 CHAPTER II. SECTION 1. MODELS OF E-ENOLATE FORMATION ...... .... ....... ... 12 SECTION 2. PATERSON ENOLATE PAPER ..... .........................