Changes in the Abundance of Danish Orchids Over the Past 30 Years
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of the Orchids of the Crimea (Orchidaceae)
J. Eur. Orch. 46 (2): 407 - 436. 2014. Alexander V. Fateryga and Karel C.A.J. Kreutz Checklist of the orchids of the Crimea (Orchidaceae) Keywords Orchidaceae, checklist of species, new nomenclature combinations, hybrids, flora of the Crimea. Summary Fateryga, A.V. & C.A.J. Kreutz (2014): Checklist of the orchids of the Crimea (Orchidaceae).- J. Eur. Orch. 46 (2): 407-436. A new nomenclature checklist of the Crimean orchids with 49 taxa and 16 hybrids is proposed. Six new taxa are added and ten taxa are excluded from the latest checklist of the Crimean vascular flora published by YENA (2012). In addition, five nomenclature changes are proposed: Epipactis persica (Soó) Nannf. subsp. taurica (Fateryga & Kreutz) Fateryga & Kreutz comb. et stat. nov., Orchis mascula (L.) L. var. wanjkovii (E. Wulff) Fateryga & Kreutz stat. nov., Anacamptis ×simorrensis (E.G. Camus) H. Kretzschmar, Eccarius & H. Dietr. nothosubsp. ticinensis (Gsell) Fateryga & Kreutz stat. nov., ×Dactylocamptis uechtritziana (Hausskn.) B. Bock ex M. Peregrym & Kuzemko nothosubsp. magyarii (Soó) Fateryga & Kreutz comb. et stat. nov., and Orchis ×beyrichii Kern. nothosubsp. mackaensis (Kreutz) Fateryga & Kreutz comb. et stat. nov. Moreover, a new variety, Limodorum abortivum (L.) Sw. var. viridis Fateryga & Kreutz var. nov. is described. Zusammenfassung Fateryga, A.V. & C.A.J. Kreutz (2014): Eine Übersicht der Orchideen der Krim (Orchidaceae).- J. Eur. Orch. 46 (2): 407-436. Eine neue nomenklatorische Liste der Orchideen der Krim mit 49 Taxa und 16 Hybriden wird vorgestellt. Sechs Arten sind neu für die Krim. Zehn Taxa, die noch bei YENA (2012) in seiner Checklist aufgelistet wurden, kommen auf der Krim nicht vor und wurden gestrichen. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Pdf of JHOS January 2012
JJoouurrnnaall of the HHAARRDDYY OORRCCHHIIDD SSOOCCIIEETTYY Vol. 9 No. 1 (63) January 2012 JOURNAL of the HARDY ORCHID SOCIETY Vol. 9 No. 1 (63) January 2012 The Hardy Orchid Society Our aim is to promote interest in the study of Native European Orchids and those from similar temperate climates throughout the world. We cover such varied aspects as field study, cultivation and propagation, photography, taxonomy and systematics, and practical conservation. We welcome articles relating to any of these subjects, which will be considered for publication by the editorial committee. Please send your submissions to the Editor, and please structure your text according to the “Advice to Authors” (see website www.hardyorchidsociety.org.uk , January 2004 Journal, Members’ Handbook or contact the Editor). Views expressed in journal arti - cles are those of their author(s) and may not reflect those of HOS. The Hardy Orchid Society Committee President: Prof. Richard Bateman, Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3DS Chairman: Celia Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Vice-Chairman: David Hughes, Linmoor Cottage, Highwood, Ringwood, Hants., BH24 3LE [email protected] Secretary: Alan Leck, 61 Fraser Close, Deeping St. James, Peterborough, PE6 8QL [email protected] Treasurer: John Wallington, 17, Springbank, Eversley Park Road, London, N21 1JH [email protected] Membership Secretary: Moira Tarrant, Bumbys, Fox Road, Mashbury, -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -
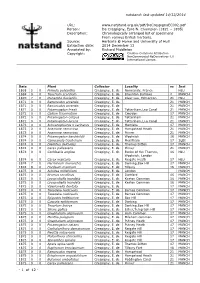
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Taxonomic Notes on Anacamptis Pyramidalis Var. Urvilleana (Orchidaceae), a Good Endemic Orchid from Malta
J. Eur. Orch. 48 (1): 19 – 28. 2016. Stephen Mifsud Taxonomic notes on Anacamptis pyramidalis var. urvilleana (Orchidaceae), a good endemic orchid from Malta Keywords Orchidaceae; Anacamptis urvilleana; Anacamptis pyramidalis; Anacamptis pyramidalis var. urvilleana; Maltese endemics; Flora of Malta; Central Mediterranean region. Summary Mifsud S. (2016): Taxonomic notes on Anacamptis pyramidalis var. urvilleana (Orchidaceae), a good endemic orchid from Malta.- J. Eur. Orch. 48 (1): 19-28. In several global plant species databases the Maltese-endemic Anacamptis urvilleana is considered as a synonym of A. pyramidalis, hence reflecting the belief of some European authors. A number of morphological differences and phenology differentiate the Maltese pyramidical orchid from A. pyramidalis. As a result, it is suggested to maintain the identity of this orchid as A. pyramidalis var. urvilleana which merits conservation treatments different from the widely distributed A. pyramidalis s. str. Zusammenfassung Mifsud S. (2016): Taxonomische Anmerkungen zu Anacamptis pyramidalis var. urvilleana (Orchidaceae), eine gute endemische Orchidee von Malta.- J. Eur. Orch. 48 (1): 19-28. In verschiedenen weltweiten Datenbanken botanischer Namen, die auch die Meinung einiger europäischer Autoren wiedergeben, wird der maltesische Endemit Anacamptis urvilleana als Synonym von A. pyramidalis geführt. Die maltesische Pyramiden-Hundswurz unterscheidet sich jedoch sowohl in einer Reihe von morphologischen Merkmalen als auch phenologisch von A. pyramidalis. Auf dieser Grundlage wird vorgeschlagen, diese Orchidee als A. pyramidalis var. urvilleana zu führen. Zu ihrem Schutz sind andere Erhaltungsmaßnahmen erforderlich als für die weitverbreitete A. pyramidalis s. str. Journal Europäischer Orchideen 48 (1): 2016. 19 1. Introduction Anacamptis urvilleana Sommier & Caruana Gatto was described in 1915 (refer Fig.1) as an endemic orchid from the Maltese islands. -

Watsonia 14 (1982), 79-100
Walsonia , 14, 79-100 (\982) 79 Book Reviews Flowers of Greece and the Balkans. A field guide. Oleg Polunin. Pp. xv+592 including 62 pages of line drawings and 21 maps, with 80 colour plates. Oxford University Press, Oxford. 1980. Price £40.00 (ISBN 0-19- 217-6269). This latest of Oleg Polunin's Field guides to the European flora presents a concise, but by no means superficial, picture of the flowering plants and conifers of the Balkan peninsula. The area that is covers takes in the whole of Greece (including the East Aegean Islands, excluded from Flora Europaea), Turkey-in-Europe, Albania, Bulgaria, Jugoslavia as far north as the River Sava, and the small portion of Romania that lies south and east of the River Danube. The author has condensed his account of the rich and varied flora of the region, together with the associated mass of published material, into a form that is attractive, comprehensible and useful to the amateur botanist. A book of this type has been badly needed, as the available floristic texts on the Balkans tend to be over 50 years old, scarce, extremely expensive and written in a foreign language, often Latin. However, the most significant attribute of this book is not that it gives access to diffuse and obscure information, but that it provides a radical alternative to popular botanical accounts of Greece which emphasize the lowland spring flora, notably the orchids and other petaloid monocots. Based on the author's many years of botanical experience and extensive travel in the region, Flowers of Greece and the Balkans demonstrates the wide range of flora and vegetation that the amateur botanist can expect to see in the Balkan peninsula. -

Orchids of Lakeland
a field guide to OrchidsLakeland Provincial Park, Provincial Recreation Area and surrounding region Written by: Patsy Cotterill Illustrated by: John Maywood Pub No: I/681 ISBN: 0-7785-0020-9 Printed 1998 Sepal Sepal Petal Petal Seed Sepal Lip (labellum) Ovary Finding an elusive (capsule) orchid nestled away in Sepal the boreal forest is a Scape thrilling experience for amateur and Petal experienced naturalists alike. This type Petal of recreation has become a passion for Column some and, for many others, a wonder- ful way to explore nature and learn Ovary Sepal (capsule) more about the living world around us. Sepal But, we also need to be careful when Lip (labellum) looking at these delicate plants. Be mindful of their fragile nature... keep Spur only memories and take only photo- graphs so that others, too, can enjoy. Corm OrchidsMany people are surprisedin Alberta to learn that orchids grow naturally in Alberta. Orchids are typically associated with tropical rain forests (where indeed, many do grow) or flower-shop purchases for special occasions, exotic, expensive and highly ornamental. Wild orchids in fact are found world-wide (except in Antarctica). They constitute Roots Tuber the second largest family of flowering plants (the Orchidaceae) after the Aster family, with upwards of 15,000 species. In Alberta, there are 26 native species. Like all Roots orchids from north temperate regions, these are terrestrial. They grow in the ground, in contrast to the majority of rain forest species which are epiphytes, that is, they grow attached to trees for support but do not derive nourishment from them. -

Cambridgeshire and Peterborough County Wildlife Sites
Cambridgeshire and Peterborough County Wildlife Sites Selection Guidelines VERSION 6.2 April 2014 CAMBRIDGESHIRE & PETERBOROUGH COUNTY WILDLIFE SITES PANEL CAMBRIDGESHIRE & PETERBOROUGH COUNTY WILDLIFE SITES PANEL operates under the umbrella of the Cambridgeshire and Peterborough Biodiversity Partnership. The panel includes suitably qualified and experienced representatives from The Wildlife Trust for Bedfordshire, Cambridgeshire, Northamptonshire; Natural England; The Environment Agency; Cambridgeshire County Council; Peterborough City Council; South Cambridgeshire District Council; Huntingdonshire District Council; East Cambridgeshire District Council; Fenland District Council; Cambridgeshire and Peterborough Environmental Records Centre and many amateur recorders and recording groups. Its aim is to agree the basis for site selection, reviewing and amending them as necessary based on the best available biological information concerning the county. © THE WILDLIFE TRUST FOR BEDFORDSHIRE, CAMBRIDGESHIRE AND NORTHAMPTONSHIRE 2014 © Appendices remain the copyright of their respective originators. All rights reserved. Without limiting the rights under copyright reserved above, no part of this publication may be reproduced, stored in any type of retrieval system or transmitted in any form or by any means (electronic, photocopying, mechanical, recording or otherwise) without the permission of the copyright owner. INTRODUCTION The Selection Criteria are substantially based on Guidelines for selection of biological SSSIs published by the Nature Conservancy Council (succeeded by English Nature) in 1989. Appropriate modifications have been made to accommodate the aim of selecting a lower tier of sites, i.e. those sites of county and regional rather than national importance. The initial draft has been altered to reflect the views of the numerous authorities consulted during the preparation of the Criteria and to incorporate the increased knowledge of the County's habitat resource gained by the Phase 1 Habitat Survey (1992-97) and other survey work in the past decade. -

Orchid Flora of the Muntele Mic (Caraş – Severin Country, Romania)
BIO LOGICA NYSSANA 7 (2) ⚫ December 2016: 107-112 Milanovici, S. ⚫ The orchid flora of the Muntele Mic… DOI: 10.5281/zenodo.2528264 7 (2) • December 2016: 107-112 12th SFSES • 16-19 June 2016, Kopaonik Mt Original Article Received: 25 June 2018 Revised: 28 Sptember 2018 Accepted: 18 November 2018* The orchid flora of the Muntele Mic (Caraş – Severin County, Romania) Sretco Milanovici Natural Science Section, Banat National Museum, Huniade Square no. 1, Timișoara City, Timiș County, Romania * E-mail: [email protected] Abstract: Milanovici, S.: The orchid flora of the Muntele Mic (Caraş – Severin County, Romania). Biologica Nyssana, 7 (2), December 2016: 107-112. Muntele Mic Mountain is located in the southwestern part of Romania and belongs to the Southern Carpathians. Although relatively small, Muntele Mic contains most of typical mountain and high-mountain habitats. The field research regarding the orchid’s family in the Muntele Mic area, have started in the summer of 2009. Owing to easy access (asphalt road that goes to the tourist center of Muntele Mic), although it is classified as part of the European Natura 2000 network (ROSCI0126 Munţii Ţarcu), the area is influenced by negative anthropogenic factors. Although considered to be a very anthropized area, the field research concluded that there are 10 species of orchids growing in this location, of which three: Gymnadenia frivaldii Hampe ex Griseb., Dactylorhiza fuchsii (Druce) Soó and Dactylorhiza saccifera (Brongn.) Soó), were not mentioned in the literature data. Key words: orchids, conservation, threats, Muntele Mic, Romania Apstrakt: Milanovici, S.: Flora orhideja planine Muntele Mic (Caraş – Severin County, Romania). -

Rare Plant Monitoring 2017
RARE PLANT MONITORING 2017 Ajuga pyramidalis Ophrys insectifera © Zoe Devlin What is it? In 2017, we decided to carry out a small pilot scheme on rare plant monitoring. Where experienced plant recorders had submitted recent casual records of rare plants to the Centre, they were asked if they would be willing to visit their rare plant population once a year during its flowering period and to count the total number of individuals present. The response to the scheme from the small number of recorders contacted has been overwhelming positive and it has resulted in very valuable data being collected in 2017. Data on the rare plant location, the count and additional information about the site is submitted online through a dedicated web portal set up by the Data Centre. The project was discussed and agreed with the NPWS. It is framed around the 2016 Vascular Plant Red List and is mainly focused on monitoring vulnerable, near threatened and rare least concern species. Why is it important? When assessing the national FAST FACTS 2017 conservation status of very rare species according to IUCN Red List methodology, it is recommended that 37 you use annual population count data. That’s the total number of rare plant Given the numbers of rare plant populations that were monitored in the species a country might have, this 2017 pilot information can be difficult to collect in any volume. This citizen science project relies on the generosity of 22 expert volunteers to ‘keep an eye’ on That’s the number of rare plant species rare populations near them and to that were monitored in 2017 submit standardised count data once a year. -

“Dynamic Speciation Processes in the Mediterranean Orchid Genus Ophrys L
“DYNAMIC SPECIATION PROCESSES IN THE MEDITERRANEAN ORCHID GENUS OPHRYS L. (ORCHIDACEAE)” Tesi di Dottorato in Biologia Avanzata, XXIV ciclo (Indirizzo Sistematica Molecolare) Universitá degli Studi di Napoli Federico II Facoltá di Scienze Matematiche, Fisiche e Naturali Dipartimento di Biologia Strutturale e Funzionale 1st supervisor: Prof. Salvatore Cozzolino 2nd supervisor: Prof. Serena Aceto PhD student: Dott. Hendrik Breitkopf 1 Cover picture: Pseudo-copulation of a Colletes cunicularius male on a flower of Ophrys exaltata ssp. archipelagi (Marina di Lesina, Italy. H. Breitkopf, 2011). 2 TABLE OF CONTENTS GENERAL INTRODUCTION CHAPTER 1: MULTI-LOCUS NUCLEAR GENE PHYLOGENY OF THE SEXUALLY DECEPTIVE ORCHID GENUS OPHRYS L. (ORCHIDACEAE) CHAPTER 2: ANALYSIS OF VARIATION AND SPECIATION IN THE OPHRYS SPHEGODES SPECIES COMPLEX CHAPTER 3: FLORAL ISOLATION IS THE MAIN REPRODUCTIVE BARRIER AMONG CLOSELY RELATED SEXUALLY DECEPTIVE ORCHIDS CHAPTER 4: SPECIATION BY DISTURBANCE: A POPULATION STUDY OF CENTRAL ITALIAN OPHRYS SPHEGODES LINEAGES CONTRIBUTION OF CO-AUTHORS ACKNOWLEDGEMENTS 3 GENERAL INTRODUCTION ORCHIDS With more than 22.000 accepted species in 880 genera (Pridgeon et al. 1999), the family of the Orchidaceae is the largest family of angiosperm plants. Recently discovered fossils document their existence for at least 15 Ma. The last common ancestor of all orchids has been estimated to exist about 80 Ma ago (Ramirez et al. 2007, Gustafsson et al. 2010). Orchids are cosmopolitan, distributed on all continents and a great variety of habitats, ranging from deserts and swamps to arctic regions. Two large groups can be distinguished: Epiphytic and epilithic orchids attach themselves with aerial roots to trees or stones, mostly halfway between the ground and the upper canopy where they absorb water through the velamen of their roots.