Industry Biosecurity Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brooklyn, Cloudland, Melsonby (Gaarraay)
BUSH BLITZ SPECIES DISCOVERY PROGRAM Brooklyn, Cloudland, Melsonby (Gaarraay) Nature Refuges Eubenangee Swamp, Hann Tableland, Melsonby (Gaarraay) National Parks Upper Bridge Creek Queensland 29 April–27 May · 26–27 July 2010 Australian Biological Resources Study What is Contents Bush Blitz? Bush Blitz is a four-year, What is Bush Blitz? 2 multi-million dollar Abbreviations 2 partnership between the Summary 3 Australian Government, Introduction 4 BHP Billiton and Earthwatch Reserves Overview 6 Australia to document plants Methods 11 and animals in selected properties across Australia’s Results 14 National Reserve System. Discussion 17 Appendix A: Species Lists 31 Fauna 32 This innovative partnership Vertebrates 32 harnesses the expertise of many Invertebrates 50 of Australia’s top scientists from Flora 62 museums, herbaria, universities, Appendix B: Threatened Species 107 and other institutions and Fauna 108 organisations across the country. Flora 111 Appendix C: Exotic and Pest Species 113 Fauna 114 Flora 115 Glossary 119 Abbreviations ANHAT Australian Natural Heritage Assessment Tool EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) NCA Nature Conservation Act 1992 (Queensland) NRS National Reserve System 2 Bush Blitz survey report Summary A Bush Blitz survey was conducted in the Cape Exotic vertebrate pests were not a focus York Peninsula, Einasleigh Uplands and Wet of this Bush Blitz, however the Cane Toad Tropics bioregions of Queensland during April, (Rhinella marina) was recorded in both Cloudland May and July 2010. Results include 1,186 species Nature Refuge and Hann Tableland National added to those known across the reserves. Of Park. Only one exotic invertebrate species was these, 36 are putative species new to science, recorded, the Spiked Awlsnail (Allopeas clavulinus) including 24 species of true bug, 9 species of in Cloudland Nature Refuge. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -
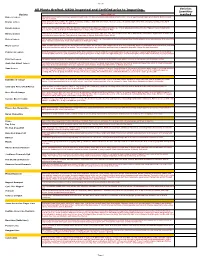
2019 Full Provisional List
Sheet1 All Plants Grafted. USDA inspected and Certified prior to Importing. Varieties Quantities Variety Description required Baboon Lemon A Brazilian lemon with very intense yellow rind and flesh. The flavour is acidic with almost a hint of lime. Tree is vigorous with large green leaves. Both tree and fruit are beautiful. Bearss Lemon 1952. Fruit closely resembles the Lisbon. Very juicy and has a high rind oil content. The leaves are a beautiful purple when first emerging, turning a nice dark green. Fruit is ready from June to December. Eureka Lemon Fruit is very juicy and highly acidic. The Eureka originated in Los Angeles, California and is one of their principal varieties. It is the "typical" lemon found in the grocery stores, nice yellow colour with typical lemon shape. Harvested November to May Harvey Lemon 1948.Having survived the disastrous deep freezes in Florida during the ’60’s and ’70’s. this varieties is known to withstand cold weather. Typical lemon shape and tart, juicy true lemon flavour. Fruit ripens in September to March. Self fertile. Zones 8A-10. Lisbon Lemon Fruit is very juicy and acic. The leaves are dense and tree is very vigorous. This Lisbon is more cold tolerant than the Eureka and is more productive. It is one of the major varieties in California. Fruit is harvested from February to May. Meyer Lemon 1908. Considered ever-bearing, the blooms are very aromatic. It is a lemon and orange hybrid. It is very cold hardy. Fruit is round with a thin rind. Fruit is juicy and has a very nice flavour, with a low acidity. -

Citrus Industry Biosecurity Plan 2015
Industry Biosecurity Plan for the Citrus Industry Version 3.0 July 2015 PLANT HEALTH AUSTRALIA | Citrus Industry Biosecurity Plan 2015 Location: Level 1 1 Phipps Close DEAKIN ACT 2600 Phone: +61 2 6215 7700 Fax: +61 2 6260 4321 E-mail: [email protected] Visit our web site: www.planthealthaustralia.com.au An electronic copy of this plan is available through the email address listed above. © Plant Health Australia Limited 2004 Copyright in this publication is owned by Plant Health Australia Limited, except when content has been provided by other contributors, in which case copyright may be owned by another person. With the exception of any material protected by a trade mark, this publication is licensed under a Creative Commons Attribution-No Derivs 3.0 Australia licence. Any use of this publication, other than as authorised under this licence or copyright law, is prohibited. http://creativecommons.org/licenses/by-nd/3.0/ - This details the relevant licence conditions, including the full legal code. This licence allows for redistribution, commercial and non-commercial, as long as it is passed along unchanged and in whole, with credit to Plant Health Australia (as below). In referencing this document, the preferred citation is: Plant Health Australia Ltd (2004) Industry Biosecurity Plan for the Citrus Industry (Version 3.0 – July 2015). Plant Health Australia, Canberra, ACT. Disclaimer: The material contained in this publication is produced for general information only. It is not intended as professional advice on any particular matter. No person should act or fail to act on the basis of any material contained in this publication without first obtaining specific and independent professional advice. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

Citrus Cavaleriei H.Lév
Volume 18: 115–119 ELOPEA Publication date: 28 May 2015 T dx.doi.org/10.7751/telopea8510 Journal of Plant Systematics plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL • ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) Lectotypification of Citrus cavaleriei H.Lév. ex Cavalerie (Rutaceae: Aurantioideae) David J. Mabberley1,2,3 and Phillip G. Kodela1 1National Herbarium of New South Wales, Royal Botanic Gardens & Domain Trust, Mrs Macquaries Road, Sydney, NSW 2000, Australia; 2Wadham College, University of Oxford, UK; 3Macquarie University, NSW 2109, Australia Abstract In the context of the elucidation of the ancestry of today’s commercial citrus crops, a lectotype is here designated for Citrus cavaleriei H.Lév. ex Cavalerie (Rutaceae, subfam. Aurantioideae), a species found in China and India, and one of the putative parents of C. ×junos Siebold ex Tanaka, the yuzu. Introduction The history of the domestication of citrus fruits is complicated by extensive hybridity between wild species, brought together by humans, and subsequent selection of apomictic clones, which make up the bulk of today’s commercial citrus crops worldwide (Mabberley 1997, 2004, 2013). The most significant globally are selections from the Citrus ×aurantium L. complex, oranges and grapefruit etc., derived from crosses between C. maxima (Burm.) Merr., the pomelo, and the mandarin from southern China, generally known as C. reticulata Blanco. The lemons and bergamots are derived from crosses between this complex and C. medica L., the citron. Even though this is well-established, there are still outstanding issues, in that no unequivocally wild populations of either C. -

Studies on the Chemistry of Australia's Native Fauna and Flora
Studies on the chemistry of Australia's native flora and fauna My first involvement in natural products chemistry was during my Honours year in chemistry at UNSW in 1964 where, like so many other chemists before me, I set to work on my bag of sawdust obtained from some Australian tree to find out what organic compounds could be extracted from it. The species involved was Sophora tomentosa and under the supervision of Associate Professor Ron Eade I obtained some isoflavone and pterocarpin derivatives. That experience whetted my interest in the chemicals contained living systems and sparked an interest in why they were there. Sophora tomentosa is a member of the Leguminosae and it is only within this family that isoflavones had been found. The work described in this thesis has been carried out at the School of Chemistry, University of New South Wales over a period of, approximately, 25 years. During this time at the University I have been responsible for providing the routine mass specti'ometry service within the School of Chemistry, though the work described herein is not part of that work. GC/MS was initiated within the School in 1969. In 1970, as a postdoctoral fellow, I used the technique for the first time in the my work on insect chemistry. After a period of 5 years at Monash University I returned to UNSW in 1976 to run the routine mass spectrometry service. My involvement with insect chemistry continued and several years later I commenced work on essential oils chemistry. Like a lot of other people who spend their lives in doing chemical research, I got into essential oils research more or less by accident, though in my case this accident was once removed. -

Genomics of the Origin and Evolution of Citrus
OPEN ARTICLE doi:10.1038/nature25447 Genomics of the origin and evolution of Citrus Guohong Albert Wu1, Javier Terol2, Victoria Ibanez2, Antonio López-García2, Estela Pérez-Román2, Carles Borredá2, Concha Domingo2, Francisco R. Tadeo2, Jose Carbonell-Caballero3, Roberto Alonso3, Franck Curk4, Dongliang Du5, Patrick Ollitrault6, Mikeal L. Roose7, Joaquin Dopazo3,8, Frederick G. Gmitter Jr5, Daniel S. Rokhsar1,9,10 & Manuel Talon2 The genus Citrus, comprising some of the most widely cultivated fruit crops worldwide, includes an uncertain number of species. Here we describe ten natural citrus species, using genomic, phylogenetic and biogeographic analyses of 60 accessions representing diverse citrus germ plasms, and propose that citrus diversified during the late Miocene epoch through a rapid southeast Asian radiation that correlates with a marked weakening of the monsoons. A second radiation enabled by migration across the Wallace line gave rise to the Australian limes in the early Pliocene epoch. Further identification and analyses of hybrids and admixed genomes provides insights into the genealogy of major commercial cultivars of citrus. Among mandarins and sweet orange, we find an extensive network of relatedness that illuminates the domestication of these groups. Widespread pummelo admixture among these mandarins and its correlation with fruit size and acidity suggests a plausible role of pummelo introgression in the selection of palatable mandarins. This work provides a new evolutionary framework for the genus Citrus. The genus Citrus -

Citrus Genomes
March 31, 2021 [AgroScience Today] Popular Article Volume 2 Issue 3 Page: 0101 – 0103 Jagannadham Prasanth Tej Kumar Scientist (Biotechnology) ICAR Central Citrus Research Institute Amravati Road Nagpur Maharashtra India - 440 033 Citrus Genomes: Thirugnanavel Anbalagan Scientist (Fruit Science) Enigma Code Breaker ICAR Central Citrus Research Institute Amravati Road Nagpur Maharashtra India - 440 033 A genome is the complete set of instructions needed to build an Anjitha George organism and allow it to grow and develop. The genome sequence Scientist (Entomology) of any organism helps to understand its evolution and lays a ICAR Central Citrus Research Institute foundation for the functional characterization means for Amravati Road understanding the genetic basis of differences between plants and Nagpur other eukaryotes, and provides the foundation for detailed Maharashtra functional characterization of plant genes. The discovery of DNA India – 440 033 as a genetic material has revolutionized the science and researchers striving to decode the genetic information. The Kiran Kumar K arrival of next generation sequencing technologies has reduced Scientist (Nematology) the time and cost required to generate draft genomes. In citrus ICAR Central Citrus Research Institute group, 9 species were sequenced, several assemblies were Amravati Road available in public domain and several were in pipeline. The Nagpur analysis of genomes unraveled the origin, evolution of citrus Maharashtra species and identification genes involved in the characters typical India – 440 033 to citrus like apomixis, vitamin C synthesis. INTRODUCTION Corresponding Authors A genome harbours the information required for the functioning of an Jagannadham Prasanth Tej Kumar organisms. The scanning of genome can reveal information leading to [email protected] a particular phenotype. -

A Phylogeny of the Rutaceae and a Biogeographic Study of Its Subfamily Aurantioideae
A phylogeny of the Rutaceae and a biogeographic study of its subfamily Aurantioideae Thomas Schwartz Supervisor Bernard Pfeil Degree project for Master of Science In Systematics and Biodiversity, Biology 60 hec Department of Plant and Environmental Science University of Gothenburg Abstract The Rutaceae classification is complex and has undergone several changes. In addition to morphological studies, phylogenetic inference using molecular data has also led to classification changes. Thus far only chloroplast data and ITS have been used, sometimes combined with morphology to infer the phylogeny. This study adds information from a low copy nuclear gene to test the existing phylogenetic hypothesis using a species tree framework. A biogeographic study was also performed on the Aurantioideae subfamily. A pilot study looked at the choice of genes, followed by testing and evaluation of several methods for extraction of Rutaceae DNA. Thereafter, a new method for efficient separation of alleles and paralogues was examined. The sequences obtained were analysed for recombination, positive selection and hybridisation. Trees for three loci (chloroplast, nuclear HYB and MDH) were made using MrBayes and BEAST, and a species tree was constructed with *BEAST. The *BEAST species tree is used as a template for a biogeographic study with the Lagrange geographic range likelihood analysis. A Bayesian biogeographic study is also performed using a Bayesian discrete biogeographical mode (an addition to BEAST). The results are then compared with previous studies, corroborating some and rejecting others. Sammanfattning Rutace-familjens struktur är komplex och föränderlig. Förutom morfologiska studier har även fylogenetiska studier använts för att få ordning i den. Hittills har man endast tittat på kloroplastgener och ribosom-DNA, i enstaka fall i kombination med morfologiska karaktärer. -

RUTACEAE 芸香科 Yun Xiang Ke Zhang Dianxiang (张奠湘)1; Thomas G
RUTACEAE 芸香科 yun xiang ke Zhang Dianxiang (张奠湘)1; Thomas G. Hartley2, David J. Mabberley3 Shrubs, trees, or sometimes herbs, sometimes scrambling or scandent, sometimes armed, with aromatic volatile oils contained in glands visible at surface of at least leaves, young branchlets, inflorescences, flower parts, fruit, or cotyledons in seed. Stipules absent [or stipular excrescences rarely present]. Leaves alternate, opposite [or whorled], simple (petiole neither apically swollen nor articulate with leaf blade), 1-foliolate (in individual specimens at least some 1-foliolate leaves with petiole apically swollen and/or articulate with leaf blade), or variously compound. Flowers bisexual or unisexual, usually 3–5-merous, actinomorphic or rarely zygomorphic, hypo- gynous [or rarely perigynous]. Perianth in 2 series, with clearly differentiated calyx and corolla or sometimes in 2 irregular series or 1 series, with ± undifferentiated tepals. Sepals distinct or connate to their full length. Petals distinct [or rarely coherent or connate for part of their length]. Stamens usually as many as or 2 × as many as petals or sometimes more numerous; filaments distinct or sometimes coherent or connate for at least part of their length; anthers introrse or sometimes latrorse, longitudinally dehiscent. Disk [rarely lack- ing] within androecium, nectariferous, flattened, annular, cup-shaped, pulvinate, or sometimes columnar, bell-shaped, conic, or hour- glass-shaped. Gynoecium of 1–5 distinct 1-loculed carpels or 2 to many partially to completely connate carpels; placentation axile [very rarely becoming parietal]; ovules 1 to many per locule. Fruit of 2–5 follicles [drupes or samaras] or a single follicle, capsule, or berry [or samara]. Seeds with relatively large embryo; endosperm present and fleshy or lacking. -

Biodiversity Summary: Burnett Mary, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations.