HUANGLONGBING and ITS VECTORS Information to Develop A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Fight Citrus Greening Disease (And It’S Not Through Genetic Engineering)
William & Mary Environmental Law and Policy Review Volume 40 (2015-2016) Issue 3 Article 7 May 2016 Saving The Orange: How to Fight Citrus Greening Disease (And It’s Not Through Genetic Engineering) Evan Feely Follow this and additional works at: https://scholarship.law.wm.edu/wmelpr Part of the Agriculture Law Commons, and the Environmental Law Commons Repository Citation Evan Feely, Saving The Orange: How to Fight Citrus Greening Disease (And It’s Not Through Genetic Engineering), 40 Wm. & Mary Envtl. L. & Pol'y Rev. 893 (2016), https://scholarship.law.wm.edu/wmelpr/vol40/iss3/7 Copyright c 2016 by the authors. This article is brought to you by the William & Mary Law School Scholarship Repository. https://scholarship.law.wm.edu/wmelpr SAVING THE ORANGE: HOW TO FIGHT CITRUS GREENING DISEASE (AND IT’S NOT THROUGH GENETIC ENGINEERING) EVAN FEELY* INTRODUCTION The orange is dying. With Florida’s citrus industry already suffer- ing from the growing skepticism of an increasingly health-conscious American public as to orange juice’s benefits,1 the emergence of citrus greening disease over the past two decades has left the orange’s long-term future very much in doubt.2 A devastating virus first documented in China roughly one hundred years ago, citrus greening disease (or “HLB”), has only migrated to Florida in the past twenty years, but has quickly made up for lost time.3 Primarily transmitted by an insect known as the Asian citrus psyllid (“ACP”), the disease has devastated Florida growers in recent years, wiping out entire groves and significantly affecting trees’ overall yield.4 This past year, Florida growers experienced their least productive harvest in forty years, and current estimates of next year’s yield are equally dismal.5 * J.D. -

Calodendrum Capense Cape Chestnut
Plant of the Week Calodendrum capense Cape Chestnut This lovely, flowering tree, Calodendrum capense, is yet another African plant which has found its way into gardens around the world. The Cape Chestnut, in spite of its name, is not related to European Chestnuts which belong in the Oak and Beech family (Fagaceae). In fact, it belongs in the Rutaceae, so is closely related to Citrus, Boronia, Eriostemon and many important Australian rainforest trees, such as Australian Teak – Flindersia australis. And the Cape Chestnut doesn’t just grow in South Africa: it is widespread through countries of eastern Africa, extending into the equatorial highlands of Kenya1. It is said that when botanist Carl Peter Thunberg, known as the “father of South African Botany”, first sighted the tree in 1772, he was so excited that he used his gun to shoot down branches so that he could examine the flowers at close hand1. In Sydney, Cape Chestnuts flower from November through to December. This is the time when we also enjoy the scarlet flowers of Illawarra Flame Trees, the soft mauve blue of Jacaranda and the golden spires of Silky Oaks. Look for clouds of soft, pink flowers on trees with rounded crowns, often in older suburbs of Sydney. Don’t forget too, that a trip on a Sydney Harbour Ferry, to Watsons Bay, Manly or to Parramatta, will give you wonderful views of these beautiful summer flowering trees. 1 Alice Notten, Kirstenbosch National Botanic Garden: http://www.plantzafrica.com/plantcd/calodendcape.htm Text and photographs: Alison Downing & Kevin Downing, 11.xii.2011, Downing Herbarium, Department of Biological Sciences . -

Fruits; Fresh Vegetables and Fresh Limes” (Opp
Trademark Trial and Appeal Board Electronic Filing System. http://estta.uspto.gov ESTTA Tracking number: ESTTA881622 Filing date: 03/07/2018 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE TRADEMARK TRIAL AND APPEAL BOARD Proceeding 91238258 Party Plaintiff Wonderful Citrus LLC Correspondence DARYA P LAUFER ESQ Address ROLL LAW GROUP PC 11444 WEST OLYMPIC BLVD LOS ANGELES, CA 90064 UNITED STATES Email: [email protected], [email protected] Submission Other Motions/Papers Filer's Name Michael M. Vasseghi Filer's email [email protected], [email protected] Signature / Michael M. Vasseghi / Date 03/07/2018 Attachments Opposition with Exhibits-reduced size.pdf(1950576 bytes ) IN THE UNITED STATES PATENT AND TRADEMARK OFFICE TRADEMARK TRIAL AND APPEAL BOARD Wonderful Citrus LLC, Opposition No. 91238258 Opposer, Application Serial No. 87/472272 v. APB, Inc. dba Vision Produce Company, Applicant. OPPOSER WONDERFUL CITRUS LLC’S OPPOSITION TO APPLICANT’S MOTION FOR JUDGMENT ON THE PLEADINGS I. INTRODUCTION Applicant moves for judgment on the pleadings (“Motion”), arguing that “there is no genuine issue as to Opposer’s lack of prior rights in a trademark that could be confusingly similar to Applicant’s Mark.” (Motion pg. 3.)1 Applicant’s Motion is not well taken. It acknowledges that Opposer has alleged exactly what it takes issue with – that Opposer has prior rights in a trademark that could be confusingly similar to Applicant’s Mark. Despite this, Applicant seeks to take issue with those allegations, implicitly contending that Opposer will be unable to prove what it has alleged. (Motion pg. 2.) This is not a proper basis for judgment on the pleadings, which must accept as true all allegations asserted in the Opposition. -

Citrus Bacterial Canker Disease and Huanglongbing (Citrus Greening)
PUBLICATION 8218 Citrus Bacterial Canker Disease and Huanglongbing (Citrus Greening) MARYLOU POLEK, Citrus Tristeza Virus Program, California Department of Food and Agriculture, Tulare; GEORGIOS VIDALAKIS, Citrus Clonal Protection Program (CCPP), Department of Plant Pathology, University of California, Riverside; and KRIS GODFREY, UNIVERSITY OF Biological Control Program, California Department of Food and Agriculture, Sacramento CALIFORNIA Division of Agriculture INTroduCTioN and Natural Resources Compared with the rest of the world, the California citrus industry is relatively free of http://anrcatalog.ucdavis.edu diseases that can impact growers’ profits. Unfortunately, exotic plant pathogens may become well established before they are recognized as such. This is primarily because some of the initial symptoms mimic other diseases, mineral deficiencies, or toxicities. In addition, development of disease symptoms caused by some plant pathogenic organisms occurs a long time after initial infection. This long latent period results in significantly delayed disease diagnosis and pathogen detection. Citrus canker (CC) and huanglong- bing (HLB, or citrus greening) are two very serious diseases of citrus that occur in many other areas of the world but are not known to occur in California. If the pathogens caus- ing these diseases are introduced into California, it will create serious problems for the state’s citrus production and nursery industries. CiTrus BACTerial CaNker Disease Citrus bacterial canker disease (CC) is caused by pathotypes or variants of the bacterium Xanthomonas axonopodis (for- merly campestris) pv. citri (Xac). This bacterium is a quaran- tine pest for many citrus-growing countries and is strictly regulated by international phytosanitary programs. Distinct pathotypes are associated with different forms of the disease (Gottwald et al. -

UNIVERSITY of CALIFORNIA RIVERSIDE Cross-Compatibility, Graft-Compatibility, and Phylogenetic Relationships in the Aurantioi
UNIVERSITY OF CALIFORNIA RIVERSIDE Cross-Compatibility, Graft-Compatibility, and Phylogenetic Relationships in the Aurantioideae: New Data From the Balsamocitrinae A Thesis submitted in partial satisfaction of the requirements for the degree of Master of Science in Plant Biology by Toni J Siebert Wooldridge December 2016 Thesis committee: Dr. Norman C. Ellstrand, Chairperson Dr. Timothy J. Close Dr. Robert R. Krueger The Thesis of Toni J Siebert Wooldridge is approved: Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS I am indebted to many people who have been an integral part of my research and supportive throughout my graduate studies: A huge thank you to Dr. Norman Ellstrand as my major professor and graduate advisor, and to my supervisor, Dr. Tracy Kahn, who helped influence my decision to go back to graduate school while allowing me to continue my full-time employment with the UC Riverside Citrus Variety Collection. Norm and Tracy, my UCR parents, provided such amazing enthusiasm, guidance and friendship while I was working, going to school and caring for my growing family. Their support was critical and I could not have done this without them. My committee members, Dr. Timothy Close and Dr. Robert Krueger for their valuable advice, feedback and suggestions. Robert Krueger for mentoring me over the past twelve years. He was the first person I met at UCR and his willingness to help expand my knowledge base on Citrus varieties has been a generous gift. He is also an amazing friend. Tim Williams for teaching me everything I know about breeding Citrus and without whom I'd have never discovered my love for the art. -

Granulation in Florida Citrus
Literature Cited harvest modulate the severity of postharvest peel pitting in citrus. J. Amer. Soc. Hort. Sci. (In press). Agusti, M., V. Almela, M. Juan, F. Alferez, F. R. Tadeo, and L. Zacarias. 2001. Lafuente, M. T. and J. M. Sala. 2002. Abscisic acid and the influence of ethyl- Histological and physiological characterization of rind breakdown of Na- ene, humidity and temperature on the incidence of postharvest rindstain- velate sweet orange. Ann. Bot. 88:422-451. ing of Navelina oranges (Citrus sinensis L. Osbeck) fruits. Postharvest Biol. Alferez, F., M. Agusti, and L. Zacarias. 2003. Postharvest rind staining in Na- Technol. 25:49-57. vel oranges is aggravated by changes in storage relative humidity: effect Petracek, P. D., L. Montalvo, H. Dou, and C. Davis. 1998. Postharvest pitting on respiration, ethylene production and water potential. Postharvest Bi- of ‘Fallglo’ tangerine. J. Amer. Soc. Hort. Sci. 123: 130-135. ol. Technol. 28:143-152. Petracek, P. D., W. F. Wardowsky, and G. E. Brown. 1995. Pitting of grapefruit Alferez, F. and J. Burns. 2004. Postharvest peel pitting at non-chilling temper- that resembles chilling injury. HortScience 30:1422-1426. atures in grapefruit is promoted by changes from low to high relative hu- Shomer, I. and Y. Erner. 1989. The nature of oleocellosis in citrus fruits. Bot. midity during storage. Postharvest Biol. Technol. 32:79-87. Gazette 50:281-288. Alferez, F., L. Zacarias, and J. Burns. 2004. Cumulative hours of low relative humidity before storage at high relative humidity and relative humidity at Proc. Fla. State Hort. Soc. 117:358-361. -

Mutation in Citrus
Mutation in Citrus MASAO NISHIURA Chief, 1st Laboratory of Fruit Tree, Okitsu Branch, Horticultural Research Station Most of the present commercial citrus varie concerning bud variations, and as a matter of ties cultivated in the world are said to have course. the more the Citrus variety is widely arisen through some kind of natural mutation. planted. the more variations are found in it. Methods of artificial vegetative propagation. In addition. certain chromosomal' changes such as grafting, cutting and layering. which were observed in some citrus. In this paper. are popularly used in fruit trees. facilitate the the bud variations found in Satsuma mandarin, conservation and accumulation of mutation. sweet orange, grapefruit. Natsudaidai and other particularly such a mutation followed by steril varieties will be mentioned in the main. ity. as it must be destined to be eliminated under sexual reproduction. Natural Gene Mutation Moreover. nucellar embryony- extra embryos. derived not from the egg cell but from somatic 1) Variation in Satsuma mandarin cells of the nucellus. are developed in the ovules Unshu-mikan or Satsuma mandarin replaced in most varieties of Citrus. and also in For the older varieties about 100 years ago, because tunella and Poncirus. This phenomenon is con of its early ripening character, superior quality sidered to have been of great advantage in and seedlessness. As Satsuma culture increas maintaining natural mutation. since early man ed, growers soon began to distinguish differences kind had no technique of vegetative propaga between Satsumas grown in various localities. tion. Pomological studies of the various Satsuma In ancient times. Citrus may have secured types by Dr. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -

Trees and Plants for Bees and Beekeepers in the Upper Mara Basin
Trees and plants for bees and beekeepers in the Upper Mara Basin Guide to useful melliferous trees and crops for beekeepers December 2017 Contents Who is this guide for? .......................................................................................................................................................................................................................................................................... 1 Introduction to the MaMaSe Project .................................................................................................................................................................................................................................................. 1 Market driven forest conservation initiatives in the Upper Mara basin ............................................................................................................................................................................................. 2 Water, apiculture, forests, trees and livelihoods ................................................................................................................................................................................................................................ 3 Types of bees ....................................................................................................................................................................................................................................................................................... 4 How this -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -
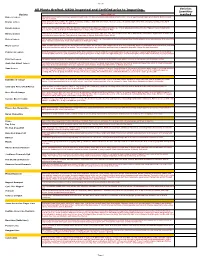
2019 Full Provisional List
Sheet1 All Plants Grafted. USDA inspected and Certified prior to Importing. Varieties Quantities Variety Description required Baboon Lemon A Brazilian lemon with very intense yellow rind and flesh. The flavour is acidic with almost a hint of lime. Tree is vigorous with large green leaves. Both tree and fruit are beautiful. Bearss Lemon 1952. Fruit closely resembles the Lisbon. Very juicy and has a high rind oil content. The leaves are a beautiful purple when first emerging, turning a nice dark green. Fruit is ready from June to December. Eureka Lemon Fruit is very juicy and highly acidic. The Eureka originated in Los Angeles, California and is one of their principal varieties. It is the "typical" lemon found in the grocery stores, nice yellow colour with typical lemon shape. Harvested November to May Harvey Lemon 1948.Having survived the disastrous deep freezes in Florida during the ’60’s and ’70’s. this varieties is known to withstand cold weather. Typical lemon shape and tart, juicy true lemon flavour. Fruit ripens in September to March. Self fertile. Zones 8A-10. Lisbon Lemon Fruit is very juicy and acic. The leaves are dense and tree is very vigorous. This Lisbon is more cold tolerant than the Eureka and is more productive. It is one of the major varieties in California. Fruit is harvested from February to May. Meyer Lemon 1908. Considered ever-bearing, the blooms are very aromatic. It is a lemon and orange hybrid. It is very cold hardy. Fruit is round with a thin rind. Fruit is juicy and has a very nice flavour, with a low acidity. -

The Taxonomy, Chorology and Reproductive Biology of Southern Afri Can Meliaceae and Ptaeroxylaceae
Bothalia 16.2: 143-168 (1986) The taxonomy, chorology and reproductive biology of southern Afri can Meliaceae and Ptaeroxylaceae F. WHITE* Keywords: chorology. Meliaceae. Ptaeroxylaceae. reproductive biology, southern Africa, taxonomy ABSTRACT Information is provided on the taxonomy, chorology and reproductive biology of 14 indigenous and two intro duced species of Meliaceae in southern Africa, and on Ptaeroxylon (Ptaeroxylaceae). Two new taxa are described: Nymanieae F. White, tribus nov. and Turraea strevi F. White & B. T. Styles, sp. nov. Nurmonia (Harms) F. White, comb, et stat. nov.. a new section of Turraea L. is created. The account complements the treatments of these families in the Flora o f southern Africa. UITTREKSEL Inligting word verskaf oor die taksonomie. chorologie en voortplantingsbiologie van 14 inheemse en twee inge- voerde spesies van Meliaceae in suidelike Afrika en oor Ptaeroxylon (Ptaeroxylaceae). Twee nuwe taksons word beskryf: Nymanieae F. White, tribus nov. en Turraea strevi F. White & B. T. Styles, sp. nov. Nurmonia (Harms) F. White, comb, et stat. nov., 'n nuwe seksie van Turraea L. word geskep. Hierdie verslag is aanvullend tot die behandelings van hierdie families in die Flora o f southern Africa. CONTENTS The position of Ptaeroxylon and Nyma nia............................................................ 163 Introduction.................................................................143 South African Trichilia: chemistry and Generic and family delimitation..................... .......144 the taxonomist's e y e .......................... 163 The position of Ptaeroxylon.................................144 Conclusions................................................... 163 The position of N ym ania.....................................144 Taxonomy as a visual a rt.............................. 163 The circumscription of Turraea..........................145 The Meliaceae and the chorology of south Notes on individual genera and species ern Africa.................................................. 164 1.