Venous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

CCM2 and CCM3 Proteins Contribute to Vasculogenesis and Angiogenesis in Human Placenta
Histol Histopathol (2009) 24: 1287-1294 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology CCM2 and CCM3 proteins contribute to vasculogenesis and angiogenesis in human placenta Gamze Tanriover1, Yasemin Seval1, Leyla Sati1, Murat Gunel2 and Necdet Demir1 1Department of Histology and Embryology, Akdeniz University, School of Medicine, Antalya, Turkey and 2 Department of Neurosurgery, Yale University, School of Medicine, New Haven, CT, USA Summary. Placenta as an ideal model to study Introduction angiogenic mechanisms have been established in previous studies. There are two processes, The placenta is a multifaceted organ that plays a vasculogenesis and angiogenesis, involved in blood critical role in maintaining and protecting the developing vessel formation during placental development. fetus. Normal development and function of the placenta Therefore, blood vessel formation is a crucial issue that requires extensive vasculogenesis and subsequent might cause vascular malformations. One of the vascular angiogenesis, in both maternal and fetal tissues. malformations is cerebral cavernous malformation Vasculogenesis is the formation of the primitive vascular (CCM) in the central nervous system, consisting of network de novo from progenitor cells, and angiogenesis endothelium-lined vascular channels without intervening is identified as the extension of blood vessels from normal brain parenchyma. Three CCM loci have been preexisting vascular structures (Demir et al., 1989, 2006; mapped as Ccm1, Ccm2, Ccm3 genes in CCM. In order Geva et al., 2002; Charnock-Jones et al., 2004). Many to investigate whether CCM proteins participate in blood factors, such as vascular endothelial growth factor vessel formation, we report here the expression patterns (VEGF), angiopoietins (Angpt-1 and -2) and their of CCM2 and CCM3 in developing and term human receptors are involved in the molecular regulation of placenta by means of immunohistochemistry and these diverse developmental steps. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -
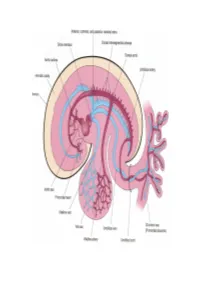
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

The Placenta
The placenta Learning module Developed by Carolyn Hammer Edited by Fabien Giroux Diagrams By Dr Julien Yockell Lelievre where indicated The placenta – Learning module Table of content 1) Introduction……………………………………………………………………….3 2) Anatomy and Physiology………………………………………………………..6 3) Roles and Functions……………………………………………………………17 4) Development and formation…………………………………..…………….…27 5) What happens after birth……………………………………………….……...34 6) What happens when things go wrong.……………………………………….36 7) Interesting facts about pregnancy………………….…………………………46 2 The placenta – Learning module Introduction 3 The placenta – Learning module What is the placenta? •The placenta is a “vascular (supplied with blood vessels) organ in most mammals that unites the fetus to the uterus of the mother. It mediates the metabolic exchanges of the developing individual through an intimate association of embryonic tissues and of certain uterine tissues, serving the functions of nutrition, respiration, and excretion.” (Online Britannica encyclopaedia) •As the fetus is in full development, it requires a certain amount of gases and nutrients to help support its growth. Because the fetus is unable to do so on its own, the placenta provides these gases and nutrients throughout pregnancy. http://health.allrefer.com/health/plac enta-abruptio-placenta.html 4 The placenta – Learning module What are the main roles of the placenta? •The placenta provides the connection between fetus and mother in order to help carry out many different functions that the growing baby is incapable to do so alone. During pregnancy, the placenta has 6 main roles to maintain good health and a good environment for the growing child: •Respiration •Nutrition •Excretion •Protection •Endocrine •Immunity 5 The placenta – Learning module Anatomy and physiology 6 The placenta – Learning module Structure •A placenta is an organ of round or oval shape that is relatively flat. -

The Structural Heterogeneity of Chorial Villi Phenotype Determined by Angiogenesis
Rom J Leg Med [19] 197-210 [2011] DOI: 10.4323/rjlm.2011.197 © 2011 Romanian Society of Legal Medicine The structural heterogeneity of chorial villi phenotype determined by angiogenesis. Implications in legal pathology Gheorghe S. Dragoi*, Petru Razvan Melinte, Daniel Zimta, Mohab El Din Mohamed _________________________________________________________________________________________ Abstract: Micro anatomic phenotype of chorial villi can be achieved only by means of a rigurous evaluation of its structural elements: trophoblast, vascular and mesenchyme. The authors proposed themselves to study the reciprocal relations between syncytiotrophoblast, fetal sinusoid capillaries and argentic collagen fiber fascicles inside chorial villi depending on angionesis process. The research was carried out on human biologic material using placenta fragments during 28-37 weeks of gestation. The authors consider that the collagen IV stereo distribution inside the vascular pedicle of the terminal villi, contributes to the stability, biodynamic and biokinematics of villi phenotype that is determined by branching or non branching angiogenesis. The personal results have a great value for stating the variability limits of terminal villi phenotype in ortology as well as in general or forensic pathology. Key Words: terminal villi, angiogenesis, collagen IV, villi phenotype he chorial villi phenotype is determined either by vasculogenesis either by angiogenesis. It Tis considered that until the end of gestation, the blood capillaries network reaches 550 km in length and 15 m2 in surface (Burton and Jauniaux, 1995) [7]. Angiogenesis plays an important role in formation and remodeling of blood vessels inside human placenta terminal villi. In the second half of gestation there is a growth acceleration and an increase in number for mature intermediate and terminal villi and for sinusoid blood capillaries (Mayhew, 2002) [22]. -

Azygos Vein System Abnormality: Case Report
Gülhane Týp Dergisi 2006; 48: 180-182 OLGU SUNUMU © Gülhane Askeri Týp Akademisi 2006 Azygos vein system abnormality: case report Necdet Kocabýyýk (*), Tunç Kutoðlu (**), Soner Albay (*), Bülent Yalçýn (*), Hasan Ozan (*) Summary Introduction Variations seen in the thoracic vein system are Abnormalities related to the azygos system are not rare (1). In a series related to the development of these veins. of 200 cases, Bergman et al. have reported the incidence of this anomaly During the dissection from the posterior medi- astinum of the 60-year-old male cadaver, it 26% (2). These abnormalities are generally explained by the embryolog- was observed that there was no complete ical development. Venous branching of the azygos vein varies (3). There accessory hemiazygos vein, and both posterior are two origins of the azygos and hemiazygos veins. By union of these intercostal veins and hemiazygos vein (above origins and regression of some parts, azygos system comes into its final T10 level) drained bilaterally to the azygos vein. Considering these types of variations is status (4). Different types of structures may occur when these veins important during imaging this region and surgi- develop. Abnormalities about azygos system and especially the variations cal operations. of the hemiazygos veins are not clearly described in the literature. In this Key words: Azygos vein, hemiazygos vein, superior vena cava, venous anomaly presentation absence of the accessory hemiazygos vein and possible causes of these types of variations are discussed in view of the embry- Özet ological development. Azigos ven sistem anomalisi: olgu sunumu Toraks ven sisteminde görülen varyasyonlar, embriyolojik olarak bu venlerin geliþimiyle ilgi- Case Report lidir. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
Endothelial Cell Dynamics in Vascular Development: Insights from Live-Imaging in Zebrafish
fphys-11-00842 July 20, 2020 Time: 12:10 # 1 REVIEW published: 22 July 2020 doi: 10.3389/fphys.2020.00842 Endothelial Cell Dynamics in Vascular Development: Insights From Live-Imaging in Zebrafish Kazuhide S. Okuda1,2 and Benjamin M. Hogan1,2,3* 1 Organogenesis and Cancer Program, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, 2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, VIC, Australia, 3 Department of Anatomy and Neuroscience, University of Melbourne, Melbourne, VIC, Australia The formation of the vertebrate vasculature involves the acquisition of endothelial cell identities, sprouting, migration, remodeling and maturation of functional vessel networks. To understand the cellular and molecular processes that drive vascular development, live-imaging of dynamic cellular events in the zebrafish embryo have proven highly informative. This review focusses on recent advances, new tools and new insights from imaging studies in vascular cell biology using zebrafish as a model system. Keywords: vasculogenesis, angiogenesis, lymphangiogenesis, anastomosis, endothelial cell, zebrafish Edited by: INTRODUCTION Elizabeth Anne Vincent Jones, KU Leuven, Belgium Vascular development is an early and essential process in the formation of a viable vertebrate Reviewed by: embryo. Blood and lymphatic vascular networks are composed of a complex mix of cell types: for Anna Rita Cantelmo, example smooth muscle cells, fibroblasts, pericytes and immune cells are intimately associated and Université Lille Nord de France, even integrated within mature vessel walls (Rouget, 1873; Horstmann, 1952; Nicosia and Madri, France 1987; Fantin et al., 2010; Gordon et al., 2010). The inner lining of the vasculature, made up of Jingjing Zhang, endothelial cells (ECs), is the earliest part of the vasculature to develop in the embryo and is Affiliated Hospital of Guangdong Medical University, China instructive in recruiting other lineages and cell types as the vascular system matures. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the