Lawrence Berkeley Laboratory UNIVERSITY of CALIFORNIA Recr:,V~Olawrence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORGANOMETAT,T,TC CHEMISTRY of URANIUM a Thesis Submitted By
ORGANOMETAT,T,TC CHEMISTRY OF URANIUM A thesis submitted by R1TN R. SIGURDSON, B.Sc. for the DEGREE of DOCTOR of PHILOSOPHY of the UNIVERSITY of LONDON Royal College of Science Imperial College of Science and Technology London, SW7 ?AY August 1976 TO MY PARENTS 3 ACKNOWLEDGEMENTS I would like to express my gratitude to Professor Geoffrey Wilkinson, F.R.S. for his guidance and enthusiastic support throughout the course of this work. Many thanks are also extended to Drs. Dick Andersen, Ernesto Carmona-Guzman and David Cole-Hamilton for their suggestionS, encouragement and advice, and to Dr. Kostas Mertis for his patient help during the first months. I am indebted to the Canadian Research Council of Canada for financial support during the past three years. 4 CONTENTS ABSTRACT 6 INTRODUCTION I. The Chemistry of Uranium(IV) 8 .II. The Chemistry of Uranium(V) 15 III. The Chemistry of Uranium(VI) 16 CHAPTER I. DILITHIUMHEXAALKYLURANATE(IV) COMPLEXES I. Introduction 19 II. Results and Discussion 27 III. Experimental 35 CHAPTER II. TRILITHIUMOCTAALKYLURANATE(V) COMPLEXES I. Introduction 54 II. Results and Discussion 55 III. Experimental 60 CHAPTER III. ADDITION COMPOUNDS OF URANIUM(VI) HEXAISO-PROPDXIDE WITH LITHIUM, MAGNESIUM AND ALUMINIUM ALKYLS I. Introduction 70 II. Results and Discussion 71 III. Experimental 77 CHAPTER IV. ORGANOMETALLIC CHEMISTRY OF ADAMANTANE I. Introduction 84 II. Results and Discussion 85 III. Experimental 87 REFERENCES 92 5 ABBREVIATIONS Me - methyl Et - ethyl Prn- normal-propyl Pri- iso-propyl Bun- normal-butyl But- iso-butyl But- tertiary-butyl Ph - phenyl CP cyclopentadienyl DME - dimethoxyethane tmed - N,N,NI,N'-tetramethylethylenediamine pmdt - N,N,Nt,N",N"-pentamethyldiethylenetriamine g.l.c. -

Sources, Effects and Risks of Ionizing Radiation
SOURCES, EFFECTS AND RISKS OF IONIZING RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2016 Report to the General Assembly, with Scientific Annexes UNITED NATIONS New York, 2017 NOTE The report of the Committee without its annexes appears as Official Records of the General Assembly, Seventy-first Session, Supplement No. 46 and corrigendum (A/71/46 and Corr.1). The report reproduced here includes the corrections of the corrigendum. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, in most cases, those that were in use at the time the data were collected or the text prepared. In other cases, however, the names have been updated, where this was possible and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.17.IX.1 ISBN: 978-92-1-142316-7 eISBN: 978-92-1-060002-6 © United Nations, January 2017. All rights reserved, worldwide. This publication has not been formally edited. Information on uniform resource locators and links to Internet sites contained in the present publication are provided for the convenience of the reader and are correct at the time of issue. The United Nations takes no responsibility for the continued accuracy of that information or for the content of any external website. -

Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility
Journal of NUCLEAR SCIENCE and TECHNOLOGY, 8 〔9〕, p. 498~502 (September 1971). Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility Tetsuhiko YOSHIMURA*, Chie MIYAKE* and Shosuke IMOTO* Received May 24, 1971 The reaction of UO2 with CCl4 vapor was carried out in a vacuum of 10-5 mmHg at 500℃ in order to prepare UCl4 of high purity. The lattice parameters were calculated from X-ray analysis to be a0=8.278±0.002, c0=7.460±0.009. The product thus ohtained was verified by chemical and X-ray analyses to be of anhydride form. The magnetic susceptibility was measured over the temperature range from liquid N2 to liquid He temperature, which revealed a deviation from the straight 1/X-T relation toward lower temperature. and that the anhydrous compound had ortho- I. INTRODUCTION rhombic symmetry. Since the same morpho- In the U-Cl system, there are known to logy may also exist for the chloride also, the exist four compounds, UCl3, UCl4, UCl5 and UCl4 is required to be of high purity. UCl6. Their chemical, physical and thermo- Table 1 Physical properties of UCl4 dynamic properties have been extensively investigated(1)(2) as well as those of the fluo- rides. Among these compounds UCl4 is used as the starting material for the synthesis of various uranous complexes, for its high solubility in many organic polar solvents such as methanol, ethanol, acetone, as reported by Selbin et al. (3)~(5) and Bagnall et al. (6) This com- pound is also used as the starting material for the preparation of UC(7), UN, US2(1)(8),USe2(1) etc. -

System Studies of Fission-Fusion Hybrid Molten Salt Reactors
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2013 SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS Robert D. Woolley University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Nuclear Engineering Commons Recommended Citation Woolley, Robert D., "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2628 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Robert D. Woolley entitled "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Nuclear Engineering. Laurence F. Miller, Major Professor We have read this dissertation and recommend its acceptance: Ronald E. Pevey, Arthur E. Ruggles, Robert M. Counce Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Robert D. -

Export Control Handbook for Chemicals
Export Control Handbook for Chemicals -Dual-use control list -Common Military List -Explosives precursors -Syria restrictive list -Psychotropics and narcotics precursors ARNES-NOVAU, X 2019 EUR 29879 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Xavier Arnés-Novau Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-785421 Filippo Sevini Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-786793 EU Science Hub https://ec.europa.eu/jrc JRC 117839 EUR 29879 Print ISBN 978-92-76-11971-5 ISSN 1018-5593 doi:10.2760/844026 PDF ISBN 978-92-76-11970-8 ISSN 1831-9424 doi:10.2760/339232 Luxembourg: Publications Office of the European Union, 2019 © European Atomic Energy Community, 2019 The reuse policy of the European Commission is implemented by Commission Decision 2011/833/EU of 12 December 2011 on the reuse of Commission documents (OJ L 330, 14.12.2011, p. 39). Reuse is authorised, provided the source of the document is acknowledged and its original meaning or message is not distorted. The European Commission shall not be liable for any consequence stemming from the reuse. -
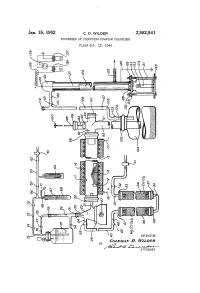
Jan. 15, 1952 C. D., WLDER 2,582,941 PROCESSES of PRODUCING URANIUM CHLORIDES Filed Oct
Jan. 15, 1952 C. D., WLDER 2,582,941 PROCESSES OF PRODUCING URANIUM CHLORIDES Filed Oct. 2, 1944 d S Q Q Q t 2. INVENTOR. 6ozEMAW Z. WZZZOER ATTORNEY . Patented Jan. 15, 1952 2,582,941 UNITED STATES PATENT OFFICE 2,582,941 PROCESSES OF PRODUCING URANUM CHELORDES - Coleman D. Wilder, Oak Ridge, Tenn., assignor to the United States. of America, as represented bysion the United States Atomic Energy Commis Application October 12, 1944, Serial No. 558,452 8 Claims. (C1. 23-145) 2. - This invention relates to the manufacture of a pating carbon tetrachloride vapor in a speedy uranium chloride product, and more particularly stream of dry air, passing the resultant mixture re of uranium pentachloride over an uranium compound heated to the neigh - - - - - a . g a substantial proportion of borhood of 550° C. whereby uranium pentachlo uranium hexachloride, by a process for chlorinat: ride is formed and vaporized into the current, ing various, compositions comprising uranium moving the resulting vaporous mixture into a compounds, with carbon tetrachloride vapor car collecting receptacle so that most of the uranium ried by a Swift current of air. chloride will condense and settle out, and send This invention has for an object the rapid pro ing the gas stream through a dust separator to duction of a uranium chloride product compris strip it of the residual uranium-containing par ing a large proportion of uranium pentachloride ticles. and various proportions of uranium hexachlo The apparatus employed comprises a device ride. for supplying carbon tetrachloride, a device for A further object of the invention is to provide supplying dry air, a flash boiler in which the a high-yield, low-loss process for the production 5 carbon tetrachloride is vaporized and mixed with of uranium pentachloride which is economical the air, a reaction chamber in which the ai and suitable for large scale production of said carbon tetrachloride admixture is reacted with chloride. -
![CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]](https://docslib.b-cdn.net/cover/9085/catalog-of-nuclear-reactor-concepts-disc-1-2699085.webp)
CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]
ANL-7092 Reactor T e lcbnology (TID-4500, 46th Ed.) 1 AEC Research and D eve lopmerit Report ARGONNENATIONAL LABORATORY 9700 South Cass Avenue Argonne, Illinois 60440 CATALOG OF NUCLEAR REACTOR CONCEPTS Part I. Homogeneous and Quasi-homogeneous Reactors Section 111. Reactors Fueled withMolten- salt Solutions . Charles E. Teeter, James A. Lecky, and John H.Martens Technical Pu'blications Department September 1965 Operated by The University of Chicago da under i Contract W - 3 1 - 109-eng -38 with the U. S. Atomic Energy Commission 445b 0311q50 ' i21 a ! i 1 4 ? i 3 TABLE OF CONTENTS --Page Preface ........................................ 5 Plan of Catalog of Nuclear Reactor Concepts. ............... 6 SECTION 111. REACTORS FUELED WITH MOLTEN-SALT SOLUTIONS. ........................... 7 Chapter 1. Introduction. .......................... 7 Chapter 2. One-region Reactors .................... 11 Chapter 3. Two-region Reactors .................... !33 c 5 . - PREFACE This report is an additional section in the Catalog of Nuclear Reactor Concepts that was begun with ANL-6892 and continued in ANL-6909. As in the previous reports, the material is divided into chapters, each with text and references, plus data sheets that cover the individual concepts. The planof the catalog, with the report numbers for the sections already issued, is given on the following page. Dr. Charles E. Teeter, formerly employed by the Chicago Oper- . ations Office at Argonne, Illinois, is now affiliated with the Southeastern Massachusetts Technological Institute, New Bedford, Mass. Through. a consultantship arrangement with Argonne National Laboratory, he is con- c tinuing to help guide the organization and cofnpilation of this catalog. J.H.M. September, 1965 , PLANOFCATALOGOFREACTORCONCEPTS Gene r a1 Introduction ANL - 6 89 2 Part I. -

SYNTHESIS and CHARACTERIZATION of a SERIES of URANIUM(IV) SPECIES: Investigating Coordination with a Redox Innocent Triamine Ligand
SYNTHESIS AND CHARACTERIZATION OF A SERIES OF URANIUM(IV) SPECIES: Investigating Coordination With a Redox Innocent Triamine Ligand Student Author Selena L. Staun earned a BS complexes containing redox-active ligands, and studies 2+ degree in chemistry with a minor in the reductive silylation of the UO2 cation. in sociology in May 2016; she graduated with department honors. She will start graduate school Suzanne Bart graduated from the to pursue a PhD in chemistry University of Delaware with a BS beginning September 2016 at the in chemistry (2001) and earned her University of California, Santa PhD from Cornell University with Barbara. While at Purdue, Staun was an undergraduate Professor Paul J. Chirik (2006). researcher in the Bart Laboratory for over 2 years and Subsequently, she was an Alexander received Purdue’s Department of Chemistry 2015 von Humboldt Postdoctoral Summer Research Award. She also found time to Fellow at the Friedrich-Alexander facilitate student learning as a Supplemental Instruction University Erlangen-Nuremberg under the direction Leader for a math course and as a teaching assistant for a of Professor Karsten Meyer. In 2008 she became an biology lab. assistant professor at Purdue University, then in 2014 she was promoted to associate professor. Her research Mentors interests include organometallic transformations mediated by organoactinide species. Bart recently won John J. Kiernicki earned AB an NSF CAREER award, and has been named a 2012 degrees in chemistry and history Cottrell Scholar and 2014 Organometallics Young from Ripon College in 2011 and Investigator Fellow. is currently working on his PhD in inorganic chemistry at Purdue University. -

Nuclear Fuel Cycle, with a Liquid Ore and Fuel: Toward Renewable Energy Cite This: Sustainable Energy Fuels, 2019, 3, 1693 Claude A
Sustainable Energy & Fuels View Article Online PAPER View Journal | View Issue Nuclear fuel cycle, with a liquid ore and fuel: toward renewable energy Cite this: Sustainable Energy Fuels, 2019, 3, 1693 Claude A. Degueldre, * Richard J. Dawson and Vesna Najdanovic-Visak To fulfill the conditions required for a nuclear renewable energy concept, one has to explore a combination of processes going from the front end of the nuclear fuel cycle to the fuel production and the energy conversion using specific fluid fuels and reactors. Extraction of uranium from a diluted fluid ore such as seawater has been studied in various countries worldwide. This extraction should be carried out parsimoniously. An extraction rate of 103 tons of U per year over centuries would not modify significantly the equilibrium concentration of uranium in the oceans (3.3 ppb). This equilibrium results from the input of 104 tons of U per year by river waters and its scavenging on the sea floor from the 1.37 Â 1018 tons of water in the oceans. For a renewable uranium extraction, the use of a specific biomass material is suggested to adsorb uranium and subsequently other transition metals. The uranium loading on the biomass would be around 100 mg per kg. After contact time, the loaded material would be dried and Creative Commons Attribution-NonCommercial 3.0 Unported Licence. burned (CO2 neutral) with heat conversion into electricity. The uranium ‘burning’ in a molten salt fast reactor helps to optimize the energy conversion by burning all actinide isotopes with an excellent yield for producing a maximum amount of thermal energy from fission and converting it into electricity. -

Depleted Uranium
WHO/SDE/PHE/01.1 English only Limited Depleted uranium Sources, Exposure and Health Effects Department of Protection of the Human Environment World Health Organization Geneva April 2001 The illustration of the cover page is extracted from Rescue Mission: Planet Earth, ã Peace Child International 1994; used by permission. ã World Health Organization 2001 This document is not issued to the general public and all rights are reserved by the World Health Organization. The document may not be reviewed, abstracted, quoted, reproduced or translated, in part or in whole, without the prior written permission of WHO. No part of this document may be stored in a retrieval system or transmitted in any form or by any means – electronic, mechanical or other without the prior written permission of WHO. The views expressed in documents by named authors are solely the responsibility of those authors. Preface Depleted uranium (DU) has been used in medical and industrial applications for decades but only since its use in military conflicts in the Gulf and the Balkans has public concern been raised about potential health consequences from exposure to it. Concerns have been particularly for peacekeeping forces, humanitarian workers and local populations living and working in areas contaminated by DU following conflict. There has been a large amount of research on the health consequences to workers in the mining and milling of uranium, and on its use in nuclear power, that enables a reasonable assessment of its impact on human health1. Since DU acts chemically in the same way as uranium, and the radiological toxicity is somewhat less than uranium, this research can be used to evaluate health risks from ingestion, inhalation and contact with DU. -

Uranium (III) Precipitation in Molten Chloride by Wet Argon Sparging Jean-François Vigier, Annabelle Laplace, Catherine Renard, Manuel Miguirditchian, Francis Abraham
Uranium (III) precipitation in molten chloride by wet argon sparging Jean-François Vigier, Annabelle Laplace, Catherine Renard, Manuel Miguirditchian, Francis Abraham To cite this version: Jean-François Vigier, Annabelle Laplace, Catherine Renard, Manuel Miguirditchian, Francis Abra- ham. Uranium (III) precipitation in molten chloride by wet argon sparging. Journal of Nuclear Materials, Elsevier, 2016, 474, pp.19-27. 10.1016/j.jnucmat.2016.03.005. cea-02386899 HAL Id: cea-02386899 https://hal-cea.archives-ouvertes.fr/cea-02386899 Submitted on 6 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Nuclear Materials 474 (2016) 19e27 Contents lists available at ScienceDirect Journal of Nuclear Materials journal homepage: www.elsevier.com/locate/jnucmat Uranium (III) precipitation in molten chloride by wet argon sparging * Jean-François Vigier a, b, , Annabelle Laplace a, Catherine Renard b, Manuel Miguirditchian a, Francis Abraham b a CEA, Nuclear Energy Division, Radiochemistry & Processes Department, F-30207 Bagnols sur Ceze, France b Unite de Catalyse et de Chimie du Solide, UCCS UMR CNRS 8181, Univ. Lille Nord de France, ENSCL-USTL, B.P. 90108, 59652 Villeneuve d'Ascq Cedex, France highlights graphical abstract Precipitation of Uranium (III) is quantitative in molten salt LiCl-CaCl2 (30e70 mol%). -

Identification of Potential Waste Processing and Waste Form Options for Molten Salt Reactors
Identification of Potential Waste Processing and Waste Form Options for Molten Salt Reactors Prepared for U.S. Department of Energy MSR Campaign B.J. Riley,(a) J. McFarlane,(b) G.D. DelCul,(b) J.D. Vienna,(a) C.I. Contescu,(b) L.M. Hay,(a) A.V. Savino,(a) H.E. Adkins,(a) (a)Pacific Northwest National Laboratory (b)Oak Ridge National Laboratory August 15, 2018 NTRD-MSR-2018-000379, PNNL-27723 Approved for public release. Distribution is unlimited. DISCLAIMER This information was prepared as an account of work sponsored by an agency of the U.S. Government. Neither the U.S. Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness, of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. References herein to any specific commercial product, process, or service by trade name, trade mark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the U.S. Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the U.S. Government or any agency thereof. Identification of Potential Waste Processing and Waste Form Options for Molten Salt Reactors iv August 15, 2018 SUMMARY The overall summary of the waste management envelope discussed in this report is represented by the diagram shown in Figure S1. The