Summaries of the Usaec Basic Research Program in Chemistry (On Site )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
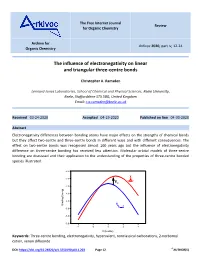
The Influence of Electronegativity on Linear and Triangular Three-Centre Bonds
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2020, part iv, 12-24 Organic Chemistry The influence of electronegativity on linear and triangular three-centre bonds Christopher A. Ramsden Lennard-Jones Laboratories, School of Chemical and Physical Sciences, Keele University, Keele, Staffordshire ST5 5BG, United Kingdom Email: [email protected] Received 03-24-2020 Accepted 04-19-2020 Published on line 04-30-2020 Abstract Electronegativity differences between bonding atoms have major effects on the strengths of chemical bonds but they affect two-centre and three-centre bonds in different ways and with different consequences. The effect on two-centre bonds was recognised almost 100 years ago but the influence of electronegativity difference on three-centre bonding has received less attention. Molecular orbital models of three-centre bonding are discussed and their application to the understanding of the properties of three-centre bonded species illustrated. -2.5 X -3.0 + Y Y Ea -3.5 -4.0 -4.5 X Binding Energy Binding + -5.0 Y Y -5.5 -6.0 -4 -2 0 2 4 h (b units) Keywords: Three-centre bonding, electronegativity, hypervalent, nonclassical carbocations, 2-norbornyl cation, xenon difluoride DOI: https://doi.org/10.24820/ark.5550190.p011.203 Page 12 ©AUTHOR(S) Arkivoc 2020, iv, 12-24 Ramsden, C. A. Table of Contents 1. Introduction 2. Linear Three-Centre Bonds (Hypervalent Bonds) (X-Y-X) X 3. Triangular Three-Centre Bonds (Y Y) 4. The Localised Bond Model of Two- and Three-Centre Bonds 5. Conclusions References -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Concentrated Stable Fluorchemical Aqueous Emulsions
Europaisches Patentamt © J European Patent Office © Publication number: 0 282 949 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION (y Application number: 88104014.1 © mt. ci.«: A61K 9/00 , A61 K 9/50 , A61 K 47/00 © Date of filing: 14.03.88 © Priority: 20.03.87 US 28521 © Applicant: AIR PRODUCTS AND CHEMICALS, INC. © Date of publication of application: Route no. 222 21.09.88 Bulletin 88/38 Trexlertown Pennsylvania 18087(US) © Designated Contracting States: © Inventor: Schweighardt, Frank Kenneth BE CH DE ES FR GB IT Li NL SE 509 Bastian Lane Rd No. 3 Allentown, PA 18104(US) Inventor: Kayhart, Charles Randall Baidy Hill Road P.O.B. 195 Alburtis, PA 18011 (US) © Representative: Dipi.-lng. Schwabe, Dr. Dr. Sandmair, Dr. Marx Stuntzstrasse 16 D-8000 MUnchen 80(DE) © Concentrated stable fluorchemical aqueous emulsions. © A stable concentrated aqueous emulsion of perfluorochemical, a phospholipid and a triglyceride of fatty acids has been demonstrated which has enhanced stability, diminished particle size and heightened tolerance by biological systems. The emulsion has utility as an oxygen transport medium, such as artificial blood. The emulsion can optionally include addition emulsifiers of SURFYNOL®SE surfactant and PLURONIC® P-105 surfactant. The emulsion is produced using an improved emulsification technique. < 03 CM CO a. Ill Xerox Copy Centre 0 282 949 CONCENTRATED STABLE FLUOROCHEMICAL AQUEOUS EMULSIONS TECHNICAL FIELD The present invention is directed to biologically acceptable oxygen transport media comprising high s concentration aqueous emulsions of perfluorochemicals in complex emulsification systems. More specifi- cally, the present invention is directed to an aqueous perfluorochemical emulsion having utility in the field of resuscitative fluids for oxygen transport and volume expansion in mammals, such as artificial or synthetic blood. -

(Title of the Thesis)*
FLUOROCARBENE, FLUOROALKYL, AND FLUORIDE COMPLEXES OF FIRST-ROW TRANSITION METALS Graham Mark Lee Thesis submitted to the Faculty of Graduate and Postdoctoral Studies University of Ottawa In partial fulfillment of the requirements for the degree of Doctor of Philosophy Ottawa-Carleton Chemistry Institute Faculty of Science University of Ottawa © Graham Mark Lee, Ottawa, Canada, 2017 Abstract Fluorinated organic compounds play important roles in our society, as these products range from life-saving pharmaceuticals and agrochemicals, to fluoropolymers with extremely high thermal and chemical stability. Although elemental fluorine (F2) is the most reactive element, some fluoro- organic compounds are chemically inert. As such, controlled reactivity of fluorine or highly- fluorinated organic fragments is a considerable, yet important challenge for synthetic chemists. Fluoro-organometallic chemistry has been studied for decades, as researchers attempt to maximize the potential of metal mediated/catalyzed processes for the synthesis of fluorinated organic molecules. Within this framework, metal fluorocarbene complexes are particularly interesting because of their highly tunable reactivity, and are proposed for use in important metathesis/polymerization reactions of perfluorinated alkenes. While considerable work is still needed to make these proposed reactions a reality, this thesis outlines contributions from our F F research group. We showed that cobalt fluorocarbene complexes CpCo(=CFR )(PPh2Me) (R = F, CF3) undergo [2+2] cycloaddition reactions with tetrafluoroethylene (TFE) and phenylacetylene to form perfluorometallacyclobutane and partially fluorinated metallacyclobutene products, respectively. For both reactions, computational studies reveal a stepwise ring-closing mechanism, which proceeds through a singlet 1,4-diradical intermediate. Next, the formation of CpCo(=CF2)(L) complexes is achieved via the direct addition of difluorocarbene, generated in situ, to a cobalt(I) precursor. -

The Radiochemistry of Tungsten
National Academy of Sciences !National Research Council NUCLEAR SCIENCE SERIES The Radiochemistry of Tungsten — ...—- L. F. C URTISS,Chairman ROBLEY D. EVANS, Vice Chairman NationalBureau ofStandards MassachusettsInstituteofTechnology J.A. DeJUREN, Secretary WestinghouseElectricCorporation C. J.BORKOWSKI J.W. IRVINE,JR. Oak RidgeNationalLaboratory MassachusettsI&tituteofTechnology ROBERT G. COCHRAN E. D. KLEMA Texas Agriculturaland Mechanical NorthwesternUniversity College W. WAYNE MEINKE SAMUEL EPSTEIN UniversityofMichigan CaliforniaInstituteofTechnology J.J.NICKSON Memorial Hospital,New York U. FANO NationalBureau ofStandards ROBERT L. PLATZMAN Laboratoirede Chimie Physique HERBERT GOLDSTEIN NuclearDevelopmentCorporationof D. M. VAN PATTER America BartolResearch Foundation LIAISON MEMBERS PAUL C. AEBERSOLD CHARLES K. REED Atomic Energy Commission U. S.Air Force J.HOWARD McMILLEN WILLIAM E. WRIGHT NationalScienceFoundation OfficeofNavalResearch SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chai~man HAROLD KIRBY UniversityofMichigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE FloridaStateUniversity Oak RidgeNationalLaboratory GEORGE A. COWAN JULIAN NIELSEN Los Alamos ScientificLaboratory HanfordLaboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG UniversityofWashington Argonne NationalLaboratory JEROME HUDIS PETER C. STEVENSON BrookhavenNationalLaboratory UniversityofCalifornia(Livermore) EARL HYDE LEO YAFFE UniversityofC slifornia(Berkeley) McGillUniversity CONSULTANTS NATHAN BALLOU JAMES DeVOE NavalRadiologicalDefenseLaboratory -

Creation and Nuclear Reaction Studies Sorption Behaviour of Short
PL9601122 PL9601121 High-Spin Nuclear Target of 178m2Hf: Creation and Nuclear Reaction Studies Yu. Ts. Oganesian1, S.A. Karamian1, Yu. P. Gangrsky1, B. Gorski1, B.N. Markov1, Z. Szeglowski2, Ch. Briangon3, O. Constantinescu3, M. Hussonnois3, J. Pinard3, R. Kulessa4, H.J. Wollersheim4, G. Graw5, J. de Boer5, G. Huber6 and H.V. Muradian7 XFLNR, JINR Dubna, Russia; 2H. Niewodniczanski Institute Nuclear Physics, Krakow, Poland; 3CSNSM, IPN, Orsay, France; 4GSI, Darmstadt, Germany; 6Miinchen University, Germany; 6Mainz University, Germany; 7Kurchatov Institute, Moscow, Russia. Investigations of the hafnium-178 isomers are a new scientific direction promising the devel- opment of a fundamental knowledge both in the field of the nuclear structure and of nuclear reactions. The completed experiments give grounds for hope of obtaining data on the electro- magnetic moments, on the mean radius and the deformation of the 178Hf nucleus in the state 16+, on the wave function structure of this state, as well as to study the influence of the target high spin on the differential cross sections of nuclear reactions, to find and investigate neutron resonances with a high spin, to obtain direct information on the density of the levels in the earlier inaccessible region of the spin and of the excitation energy, to measure directly the parameters of a giant dipole resonance based on the high spin state and to clarify in detail the role of the structure hindrances in nuclear reactions. Sorption Behaviour of Short-Lived W and Hf Isotopes on Ion Exchangers from HC1/HF Solutions in Fast On-Line Experiments D. Schumann1, R. Dressier1, S. Fischer1, St. -

Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology
Physical Science International Journal 15(2): 1-25, 2017; Article no.PSIJ.34889 ISSN: 2348-0130 Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology Timashev Serge 1,2* 1Karpov Institute of Physical Chemistry, Moscow, Russia. 2National Research Nuclear University MEPhI, Moscow, Russia. Author’s contribution The sole author designed, analyzed and interpreted and prepared the manuscript. Article Information DOI: 10.9734/PSIJ/2017/34889 Editor(s): (1) Prof. Yang-Hui He, Professor of Mathematics, City University London, UK And Chang-Jiang Chair Professor in Physics and Qian-Ren Scholar, Nan Kai University, China & Tutor and Quondam-Socius in Mathematics, Merton College, University of Oxford, UK. (2) Roberto Oscar Aquilano, School of Exact Science, National University of Rosario (UNR),Rosario, Physics Institute (IFIR)(CONICET-UNR), Argentina. Reviewers: (1) Alejandro Gutiérrez-Rodríguez, Universidad Autónoma de Zacatecas, Mexico. (2) Arun Goyal, Delhi University, India. (3) Stanislav Fisenko, Moscow State Linguistic University, Russia. Complete Peer review History: http://www.sciencedomain.org/review-history/20031 Received 17 th June 2017 Accepted 8th July 2017 Original Research Article th Published 13 July 2017 ABSTRACT A hypothesis of the existence of metastable states for nuclear matter with a locally shaken-up nucleonic structure of the nucleus, was proposed earlier. Such states are initiated by inelastic scattering of electrons by nuclei along the path of weak nuclear interaction. The relaxation of such nuclei is also determined by weak interactions. The use of the hypothesis makes it possible to physically interpret a rather large group of experimental data on the initiation of low energy nuclear reactions (LENRs) and the acceleration of radioactive α- and β-decays in a low-temperature plasma. -
ALB Materials Inc Product List.Xlsx
ALB Materials Inc 2360 Corporate Circle. Suite 400 Website: www.albmaterials.com Henderson, NV 89074-7739 E-mail: [email protected] ALB Rare Earth Materials Products List Item No. Product Name Formula CAS# Purity ALB-AL2113 Scandium (2%)- Aluminum Master Alloy (Sc-Al) Sc-Al [113413-85-7] [2%Sc+98%Al] ALB-ME21 Scandium (Sc) Metal Sc [7440-20-2] [99.9%-99.999%] ALB-ME21-G Scandium (Sc) Metal Granules Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-R Scandium (Sc) Metal Rods Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-S Scandium (Sc) Metal Sheets Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME39 Yttrium (Y) Metal Y [7440-65-5] [99.9%-99.99%] ALB-ME39-G Yttrium (Y) Metal Granules Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-R Yttrium (Y) Metal Rods Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-S Yttrium (Y) Metal Sheets Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME57 Lanthanum (La) Metal La [7439-91-0] [99%-99.95%] ALB-ME57-G Lanthanum (La) Metal Granules La [7439-91-0] [99%, 99.9%REM] ALB-ME57-R Lanthanum (La) Metal Rods La [7439-91-0] [99%, 99.9%REM] ALB-ME57-S Lanthanum (La) Metal Sheets La [7439-91-0] [99%, 99.9%REM] ALB-ME58 Cerium (Ce) Metal Ce [7440-45-1] [99%-99.9%] ALB-ME58-G Cerium (Ce) Metal Granules Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-R Cerium (Ce) Metal Rods Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-S Cerium (Ce) Metal Sheets Ce [7440-45-1] [99%, 99.9%REM] ALB-ME59 Praseodymium (Pr) Metal Pr [7440-10-0] [99%-99.9%] ALB-ME59-G Praseodymium (Pr) Metal Granules Pr [7440-10-0] [99.9%, 99.99%REM] ALB-ME59-R Praseodymium (Pr) Metal Rods Pr [7440-10-0] -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Problems for Chapter 17
Molecular Modeling Problems Chapter 17 1. Argon Compounds? One of the major advantages of calculation over experiment is that “reality does not get in the way”. It is no harder to investigate the properties of labile compounds that may be difficult to isolate and characterize experimentally than it is to investigate those of stable compounds. In fact, it is possible to say whether experimental characterization can ever be achieved, that is, if the molecule of interest is actually an energy minimum. Noble gas compounds illustrate this. While xenon compounds are now numerous and a few compounds of krypton have now been reported, no argon compounds have been isolated or characterized. Do argon analogues of known xenon and krypton compounds actually exist (in the sense that they represent energy minima)? If so, do they exhibit similar geometries and charge distribution as their analogues? Obtain equilibrium geometries for argon difluoride, krypton difluoride and xenon difluoride using the Hartree-Fock 3-21G model. Start from bent structures even though. KrF2 and XeF2 are known to be linear (and ArF2 might very well be assumed to be linear as well). While the geometry optimization is able to move from a non-linear to a linear structure, it cannot do the reverse. Follow each optimization by an infrared spectrum calculation to tell you whether or not the structure is actually an energy minimum. Are KrF2 and XeF2 linear molecules? Is the calculated Kr-F bond distance in reasonable accord with the experimental value of 1.89Ǻ (the bond distance in XeF2 linear is not known)? Is ArF2 an energy minimum? Is it linear? If ArF2 is an energy minimum, is dissociation to Ar and F2 endothermic or exothermic? Are the corresponding dissociations of KrF2 and XeF2 endothermic or exothermic? Obtain electrostatic potential maps for the three compounds and display side by side on screen (and on the same scale). -

Standard Thermodynamic Properties of Chemical
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ∆ ° –1 ∆ ° –1 ° –1 –1 –1 –1 Molecular fH /kJ mol fG /kJ mol S /J mol K Cp/J mol K formula Name Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Ac Actinium 0.0 406.0 366.0 56.5 188.1 27.2 20.8 Ag Silver 0.0 284.9 246.0 42.6 173.0 25.4 20.8 AgBr Silver(I) bromide -100.4 -96.9 107.1 52.4 AgBrO3 Silver(I) bromate -10.5 71.3 151.9 AgCl Silver(I) chloride -127.0 -109.8 96.3 50.8 AgClO3 Silver(I) chlorate -30.3 64.5 142.0 AgClO4 Silver(I) perchlorate -31.1 AgF Silver(I) fluoride -204.6 AgF2 Silver(II) fluoride -360.0 AgI Silver(I) iodide -61.8 -66.2 115.5 56.8 AgIO3 Silver(I) iodate -171.1 -93.7 149.4 102.9 AgNO3 Silver(I) nitrate -124.4 -33.4 140.9 93.1 Ag2 Disilver 410.0 358.8 257.1 37.0 Ag2CrO4 Silver(I) chromate -731.7 -641.8 217.6 142.3 Ag2O Silver(I) oxide -31.1 -11.2 121.3 65.9 Ag2O2 Silver(II) oxide -24.3 27.6 117.0 88.0 Ag2O3 Silver(III) oxide 33.9 121.4 100.0 Ag2O4S Silver(I) sulfate -715.9 -618.4 200.4 131.4 Ag2S Silver(I) sulfide (argentite) -32.6 -40.7 144.0 76.5 Al Aluminum 0.0 330.0 289.4 28.3 164.6 24.4 21.4 AlB3H12 Aluminum borohydride -16.3 13.0 145.0 147.0 289.1 379.2 194.6 AlBr Aluminum monobromide -4.0 -42.0 239.5 35.6 AlBr3 Aluminum tribromide -527.2 -425.1 180.2 100.6 AlCl Aluminum monochloride -47.7 -74.1 228.1 35.0 AlCl2 Aluminum dichloride -331.0 AlCl3 Aluminum trichloride -704.2 -583.2 -628.8 109.3 91.1 AlF Aluminum monofluoride -258.2 -283.7 215.0 31.9 AlF3 Aluminum trifluoride -1510.4 -1204.6 -1431.1 -1188.2 66.5 277.1 75.1 62.6 AlF4Na Sodium tetrafluoroaluminate -

Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing
Nuclear Safety NEA/ CSNI/R(2019)9/ADD1 May 2019 www.oecd-nea.org Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing Appendix C NEA/CSNI/R(2019)9/ADD1 The non-radiological risks involving dangerous chemicals in nuclear fuel cycle facilities - French framework regulation - Youcef HEMIMOU, Brice DELIME, Raffaello AMOROSI, Josquin VERNON French Nuclear Safety Authority 15, rue Louis Lejeune CS70013 – 92541 Montrouge Cedex [email protected] Accidents involving hazardous chemicals pose a significant threat to the population and the environment. Among nuclear facilities, this threat applies in particular to the fuel cycle facilities. As a consequence, the activities related to hazardous chemicals are covered by a legal framework that, depending on the nature of the activity and the associated risks, aims to guarantee that, they will not be likely to be detrimental to safety, public health and environment [1]. The aim of this article is to present a summary of the main regulations involving dangerous chemicals in nuclear fuel cycle facilities that have to be taken into account in the safety demonstration. The latter must prove that the risks of an accident - radiological or not - and the scale of its consequences, given the current state of knowledge, practices and the vulnerability of the installation environment, are as low as possible under acceptable economic conditions. Key words : nuclear fuel cycle facilities, safety demonstration, principle of defence in depth, deterministic approach, probabilistic approach, dangerous chemicals, non-radiological risks, Seveso Directive, domino effects. 1. THE DANGEROUS SUBSTANCES IN NUCLEAR FUEL CYCLE FACILITIES 1.1.