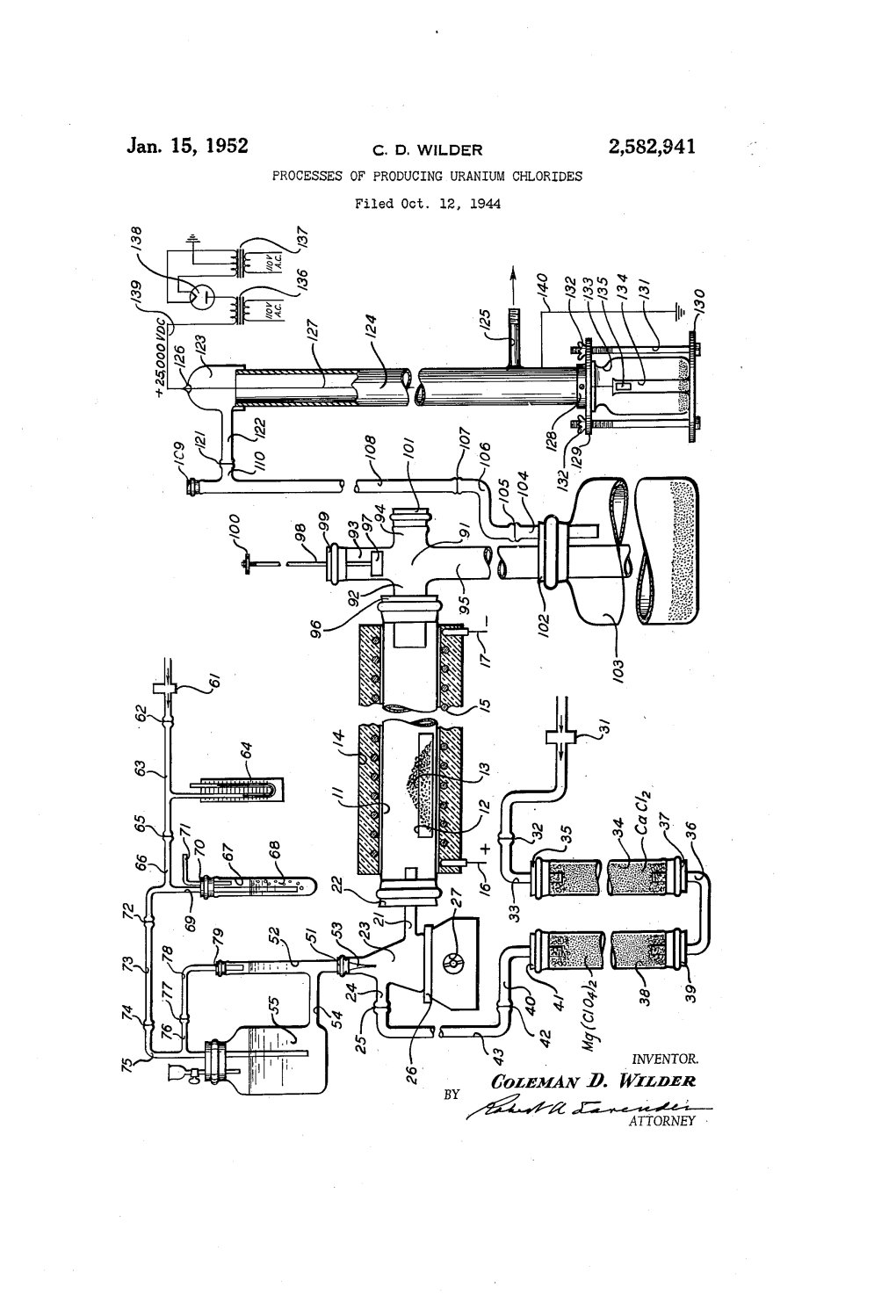
Jan. 15, 1952 C. D., WLDER 2,582,941 PROCESSES of PRODUCING URANIUM CHLORIDES Filed Oct
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORGANOMETAT,T,TC CHEMISTRY of URANIUM a Thesis Submitted By
ORGANOMETAT,T,TC CHEMISTRY OF URANIUM A thesis submitted by R1TN R. SIGURDSON, B.Sc. for the DEGREE of DOCTOR of PHILOSOPHY of the UNIVERSITY of LONDON Royal College of Science Imperial College of Science and Technology London, SW7 ?AY August 1976 TO MY PARENTS 3 ACKNOWLEDGEMENTS I would like to express my gratitude to Professor Geoffrey Wilkinson, F.R.S. for his guidance and enthusiastic support throughout the course of this work. Many thanks are also extended to Drs. Dick Andersen, Ernesto Carmona-Guzman and David Cole-Hamilton for their suggestionS, encouragement and advice, and to Dr. Kostas Mertis for his patient help during the first months. I am indebted to the Canadian Research Council of Canada for financial support during the past three years. 4 CONTENTS ABSTRACT 6 INTRODUCTION I. The Chemistry of Uranium(IV) 8 .II. The Chemistry of Uranium(V) 15 III. The Chemistry of Uranium(VI) 16 CHAPTER I. DILITHIUMHEXAALKYLURANATE(IV) COMPLEXES I. Introduction 19 II. Results and Discussion 27 III. Experimental 35 CHAPTER II. TRILITHIUMOCTAALKYLURANATE(V) COMPLEXES I. Introduction 54 II. Results and Discussion 55 III. Experimental 60 CHAPTER III. ADDITION COMPOUNDS OF URANIUM(VI) HEXAISO-PROPDXIDE WITH LITHIUM, MAGNESIUM AND ALUMINIUM ALKYLS I. Introduction 70 II. Results and Discussion 71 III. Experimental 77 CHAPTER IV. ORGANOMETALLIC CHEMISTRY OF ADAMANTANE I. Introduction 84 II. Results and Discussion 85 III. Experimental 87 REFERENCES 92 5 ABBREVIATIONS Me - methyl Et - ethyl Prn- normal-propyl Pri- iso-propyl Bun- normal-butyl But- iso-butyl But- tertiary-butyl Ph - phenyl CP cyclopentadienyl DME - dimethoxyethane tmed - N,N,NI,N'-tetramethylethylenediamine pmdt - N,N,Nt,N",N"-pentamethyldiethylenetriamine g.l.c. -

Sources, Effects and Risks of Ionizing Radiation
SOURCES, EFFECTS AND RISKS OF IONIZING RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2016 Report to the General Assembly, with Scientific Annexes UNITED NATIONS New York, 2017 NOTE The report of the Committee without its annexes appears as Official Records of the General Assembly, Seventy-first Session, Supplement No. 46 and corrigendum (A/71/46 and Corr.1). The report reproduced here includes the corrections of the corrigendum. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, in most cases, those that were in use at the time the data were collected or the text prepared. In other cases, however, the names have been updated, where this was possible and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.17.IX.1 ISBN: 978-92-1-142316-7 eISBN: 978-92-1-060002-6 © United Nations, January 2017. All rights reserved, worldwide. This publication has not been formally edited. Information on uniform resource locators and links to Internet sites contained in the present publication are provided for the convenience of the reader and are correct at the time of issue. The United Nations takes no responsibility for the continued accuracy of that information or for the content of any external website. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility
Journal of NUCLEAR SCIENCE and TECHNOLOGY, 8 〔9〕, p. 498~502 (September 1971). Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility Tetsuhiko YOSHIMURA*, Chie MIYAKE* and Shosuke IMOTO* Received May 24, 1971 The reaction of UO2 with CCl4 vapor was carried out in a vacuum of 10-5 mmHg at 500℃ in order to prepare UCl4 of high purity. The lattice parameters were calculated from X-ray analysis to be a0=8.278±0.002, c0=7.460±0.009. The product thus ohtained was verified by chemical and X-ray analyses to be of anhydride form. The magnetic susceptibility was measured over the temperature range from liquid N2 to liquid He temperature, which revealed a deviation from the straight 1/X-T relation toward lower temperature. and that the anhydrous compound had ortho- I. INTRODUCTION rhombic symmetry. Since the same morpho- In the U-Cl system, there are known to logy may also exist for the chloride also, the exist four compounds, UCl3, UCl4, UCl5 and UCl4 is required to be of high purity. UCl6. Their chemical, physical and thermo- Table 1 Physical properties of UCl4 dynamic properties have been extensively investigated(1)(2) as well as those of the fluo- rides. Among these compounds UCl4 is used as the starting material for the synthesis of various uranous complexes, for its high solubility in many organic polar solvents such as methanol, ethanol, acetone, as reported by Selbin et al. (3)~(5) and Bagnall et al. (6) This com- pound is also used as the starting material for the preparation of UC(7), UN, US2(1)(8),USe2(1) etc. -
NOI to Prepare an Environmental Impact Statement For
Federal Register / Vol. 64, No. 34 / Monday, February 22, 1999 / Notices 8553 reauthorization of OERI and will hear DEPARTMENT OF ENERGY Orders are summarized in the attached committee and officers' reports. A final appendix. [FE Docket Nos. 99±01±NG, 99±03±NG, 99± agenda will be available from the Board 02±NG, 99±04±NG, 92±24±NG, 99±05±NG, These Orders may be found on the FE office on March 10, 1999, and will be and 99±06±NG] web site at http://www.fe.doe.gov., or posted on the Board's web site, http:// on the electronic bulletin board at (202) www.ed.gov/offices/OERI/NERPPB/. Office of Fossil Energy; Orders 586±7853. Granting and Transferring Records are kept of all Board They are also available for inspection Authorizations To Import and/or Export proceedings and are available for public and copying in the Office of Natural Gas Natural Gas inspection at the office of the National & Petroleum Import & Export Activities, Educational Research Policy and OGE Energy Resources, Inc., National Fuel Docket Room 3E±033, Forrestal Priorities Board, Suite 100, 80 F St., Gas Distribution Corporation, Renaissance Building, 1000 Independence Avenue, NW, Washington, D.C. 20208±7564. Energy (U.S.) Inc., Selkirk Cogen Partners, SW, Washington, DC 20585, (202) 586± L.P., Coral Energy Resources, L.P. (Successor 9478. The Docket Room is open between Dated: February 16, 1999. to Salmon Resources Ltd.), Transco Energy the hours of 8:00 a.m. and 4:30 p.m., Eve M. Bither, Marketing Company, and Petro-Canada Hydrocarbons Inc. -

System Studies of Fission-Fusion Hybrid Molten Salt Reactors
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2013 SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS Robert D. Woolley University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Nuclear Engineering Commons Recommended Citation Woolley, Robert D., "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2628 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Robert D. Woolley entitled "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Nuclear Engineering. Laurence F. Miller, Major Professor We have read this dissertation and recommend its acceptance: Ronald E. Pevey, Arthur E. Ruggles, Robert M. Counce Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Robert D. -

Export Control Handbook for Chemicals
Export Control Handbook for Chemicals -Dual-use control list -Common Military List -Explosives precursors -Syria restrictive list -Psychotropics and narcotics precursors ARNES-NOVAU, X 2019 EUR 29879 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Xavier Arnés-Novau Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-785421 Filippo Sevini Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-786793 EU Science Hub https://ec.europa.eu/jrc JRC 117839 EUR 29879 Print ISBN 978-92-76-11971-5 ISSN 1018-5593 doi:10.2760/844026 PDF ISBN 978-92-76-11970-8 ISSN 1831-9424 doi:10.2760/339232 Luxembourg: Publications Office of the European Union, 2019 © European Atomic Energy Community, 2019 The reuse policy of the European Commission is implemented by Commission Decision 2011/833/EU of 12 December 2011 on the reuse of Commission documents (OJ L 330, 14.12.2011, p. 39). Reuse is authorised, provided the source of the document is acknowledged and its original meaning or message is not distorted. The European Commission shall not be liable for any consequence stemming from the reuse. -

This Generic Chemical Compatibility Chart Is Offered Only As a General
Chemical Compatibility Chart This Generic Chemical Compatibility Chart is offered only as a general guide, indicating the suitability of various elastomers for service in these chemicals and fluids. The ratings are based, for the most part, on published literature of various polymer suppliers, rubber manufacturers, and in some cases, the considered opinion of experienced compounders. We cannot guarantee their accuracy nor assume any responsibility for use thereof. Therefore, we recommend that you always TEST your materials for your particular application before moving to production. www.GlobalORing.com 4250 N. Sam Houston Parkway E, Suite 100, Houston, TX 77032 Phone: 832-448-5550 / 877-448-5550 Fax: 832-448-5551 / 877-448-5551 Chemical Compatibility Chart Polymer Comparison Temperature Durometer Media Resistance ASTM Range (°F) Range Abbr Low High Low High Oil Fuel Acid Alkali Ozone Steam Brake Weather ACM -40 400 50 80 E P – G P -F G - E G - E P P E AEM -50 350 40 90 F P – G P – F P – F E P P E AU -65 250 40 90 G P – G P – G P – F E P P E CIIR -50 300 60 70 P P F – E G - E E G – E G F CM -60 300 80 80 G – E P – F G – E E E P - F G E CR -80 220 30 90 F – G P – F F – G G – P G – E P – G F P - G CSM -65 275 50 80 E F G – E G – E E – O P – G F E – O ECO -80 to -15 275 to 325 50 90 E G - E P - G P – G G – E F – G P G – E EPDM -75 350 40 90 P P G G G - E E G – E E EU -65 250 40 90 G P – G P – G P – F E P P E FEPM 0 450 to 500 70 90 E P - F F - G E E G - E F – E E FKM -20 to 20 500 50 90 E E G - E P - G O P – G F E FVMQ -110 to -90 450 40 -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -
![CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]](https://docslib.b-cdn.net/cover/9085/catalog-of-nuclear-reactor-concepts-disc-1-2699085.webp)
CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]
ANL-7092 Reactor T e lcbnology (TID-4500, 46th Ed.) 1 AEC Research and D eve lopmerit Report ARGONNENATIONAL LABORATORY 9700 South Cass Avenue Argonne, Illinois 60440 CATALOG OF NUCLEAR REACTOR CONCEPTS Part I. Homogeneous and Quasi-homogeneous Reactors Section 111. Reactors Fueled withMolten- salt Solutions . Charles E. Teeter, James A. Lecky, and John H.Martens Technical Pu'blications Department September 1965 Operated by The University of Chicago da under i Contract W - 3 1 - 109-eng -38 with the U. S. Atomic Energy Commission 445b 0311q50 ' i21 a ! i 1 4 ? i 3 TABLE OF CONTENTS --Page Preface ........................................ 5 Plan of Catalog of Nuclear Reactor Concepts. ............... 6 SECTION 111. REACTORS FUELED WITH MOLTEN-SALT SOLUTIONS. ........................... 7 Chapter 1. Introduction. .......................... 7 Chapter 2. One-region Reactors .................... 11 Chapter 3. Two-region Reactors .................... !33 c 5 . - PREFACE This report is an additional section in the Catalog of Nuclear Reactor Concepts that was begun with ANL-6892 and continued in ANL-6909. As in the previous reports, the material is divided into chapters, each with text and references, plus data sheets that cover the individual concepts. The planof the catalog, with the report numbers for the sections already issued, is given on the following page. Dr. Charles E. Teeter, formerly employed by the Chicago Oper- . ations Office at Argonne, Illinois, is now affiliated with the Southeastern Massachusetts Technological Institute, New Bedford, Mass. Through. a consultantship arrangement with Argonne National Laboratory, he is con- c tinuing to help guide the organization and cofnpilation of this catalog. J.H.M. September, 1965 , PLANOFCATALOGOFREACTORCONCEPTS Gene r a1 Introduction ANL - 6 89 2 Part I. -
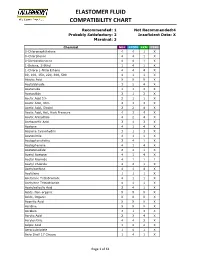
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

SYNTHESIS and CHARACTERIZATION of a SERIES of URANIUM(IV) SPECIES: Investigating Coordination with a Redox Innocent Triamine Ligand
SYNTHESIS AND CHARACTERIZATION OF A SERIES OF URANIUM(IV) SPECIES: Investigating Coordination With a Redox Innocent Triamine Ligand Student Author Selena L. Staun earned a BS complexes containing redox-active ligands, and studies 2+ degree in chemistry with a minor in the reductive silylation of the UO2 cation. in sociology in May 2016; she graduated with department honors. She will start graduate school Suzanne Bart graduated from the to pursue a PhD in chemistry University of Delaware with a BS beginning September 2016 at the in chemistry (2001) and earned her University of California, Santa PhD from Cornell University with Barbara. While at Purdue, Staun was an undergraduate Professor Paul J. Chirik (2006). researcher in the Bart Laboratory for over 2 years and Subsequently, she was an Alexander received Purdue’s Department of Chemistry 2015 von Humboldt Postdoctoral Summer Research Award. She also found time to Fellow at the Friedrich-Alexander facilitate student learning as a Supplemental Instruction University Erlangen-Nuremberg under the direction Leader for a math course and as a teaching assistant for a of Professor Karsten Meyer. In 2008 she became an biology lab. assistant professor at Purdue University, then in 2014 she was promoted to associate professor. Her research Mentors interests include organometallic transformations mediated by organoactinide species. Bart recently won John J. Kiernicki earned AB an NSF CAREER award, and has been named a 2012 degrees in chemistry and history Cottrell Scholar and 2014 Organometallics Young from Ripon College in 2011 and Investigator Fellow. is currently working on his PhD in inorganic chemistry at Purdue University.