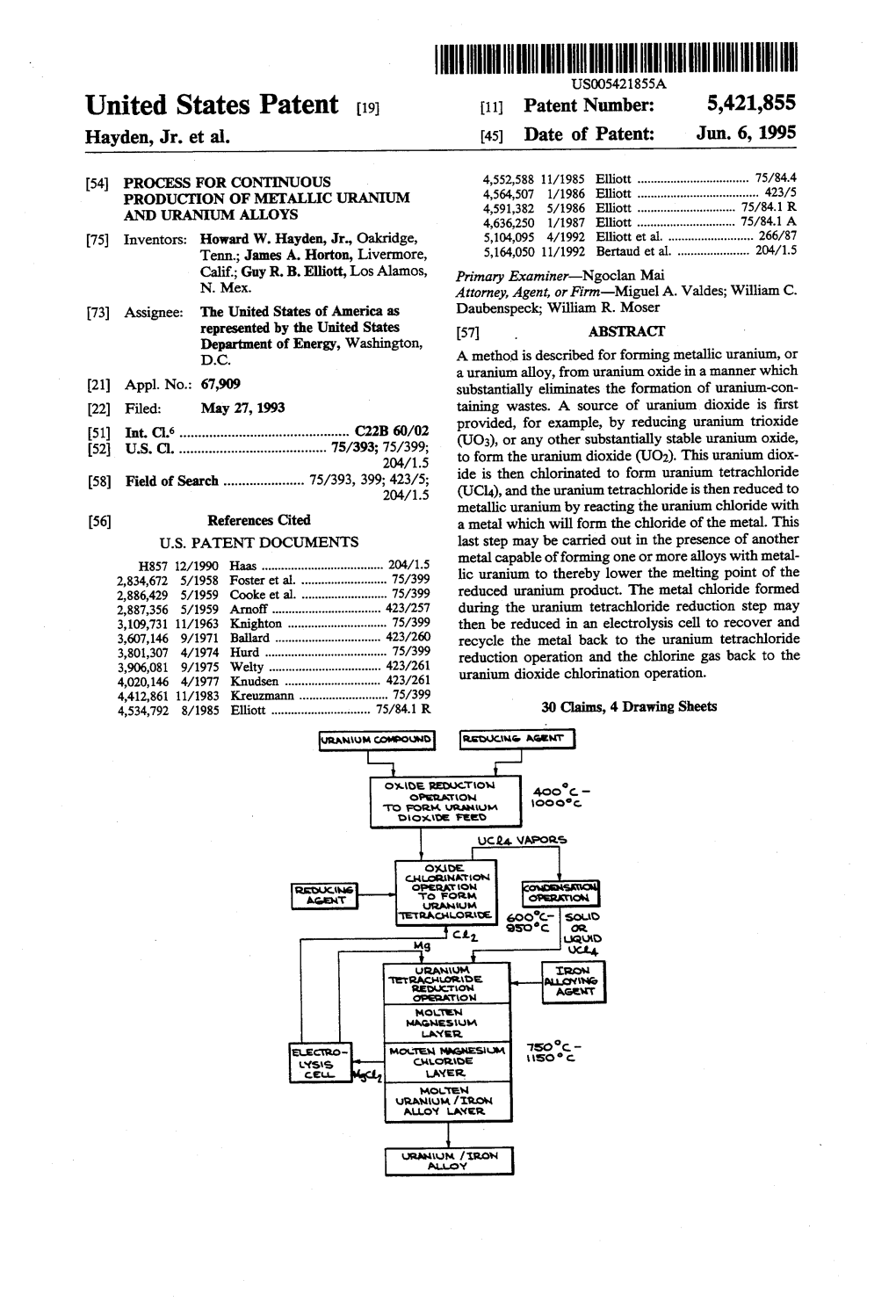
2071 to Form the Uranium Dioxide (UO2). This Uranium Diox 58) Field of Search
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Pe, Ph, and Carbonate on the Solubility of UO2 and Uraninite Under Nominally Reducing Conditions
Geochimica et Cosmochimica Acta, Vol. 62, No. 13, pp. 2223–2231, 1998 Copyright © 1998 Elsevier Science Ltd Pergamon Printed in the USA. All rights reserved 0016-7037/98 $19.00 1 .00 PII S0016-7037(98)00140-9 The role of pe, pH, and carbonate on the solubility of UO2 and uraninite under nominally reducing conditions 1 1 1 1 2 2 3, IGNASI CASAS, JOAN DE PABLO, JAVIER GIMENEZ´ , M. ELENA TORRERO, JORDI BRUNO, ESTHER CERA, ROBERT J. FINCH, * and 3,† RODNEY C. EWING 1Department of Chemical Engineering, Polytechnic University of Catalunya, Barcelona 08028, Spain 2QuantiSci SL, Parc Tecnolo`gic del Valle`s, Cerdanyola 08290, Spain 3Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, New Mexico 87131, USA (Received August 14, 1997; accepted in revised form March 26, 1998) Abstract—Experimental data obtained from uranium dioxide solubility studies as a function of pH and under nominally reducing conditions in a 0.008 mol dm23 perchlorate medium and in a 1 mol dm23 chloride solution are presented. The solubility of extensively characterized uraninite samples from Cigar Lake (Canada), Jachymov (Czech Republic), and Oklo (Gabon) was determined in a solution matching the composition of a groundwater associated with granitic terrain. The redox potential of the test solution was monitored throughout the experimental period. The results obtained were modeled using aqueous formation constants compiled by the NEA, using stability constants corrected to appropriate ionic strengths. The solubility curves have been adjusted by calculating the 1 N value of Ks4 (UO2(s) 2H2O U(OH)4(aq)) that gave the best fit with the experimental data. -

Depleted Uranium Hexafluoride: Waste Or Resource?
UCRGJC-120397 PREPRINT Depleted Uranium Hexafluoride: Waste or Resource? N. Schwertz J. Zoller R Rosen S. Patton C. Bradley A. Murray This paper was prepared for submittal to the Global ‘95 International Conference on Evaluation of Emerging Nuclear Fuel Cycle Systems Versailles, France September 11-14,1995 July 1995 This isa preprint of apaper intended for publication in a jaurnal orproceedings. Since changes may be made before publication, this preprint is made available with the understanding that it will not be cited or reproduced without the permiasion of the anthor. DISCLAIMER This document was prepared as an account of work sponsored by an agency of the United Stat= Government. Neither theunited States Governmentmor theuniversity of California nor any oftheir employees, makes any warranty, express or implied, or assumesanylegalliabilityorrespomibility forthe accuracy,completeness,orusefuin~ of any information, apparatus, pduct, or process disdosed, or represents that its use wouldnotinfringe privatelyowned rights. Referencehemin to anyspe&c commercial prodocis, proms, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constituteor imply its endorsement, reconunendation, or favoring by the United States Government or the University of California. The views and opinions of authors expressed herein do not necessady state or reflect those of the United States Government or the University of California, and shall not be used for adveltising or product endorsement purposes. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document . DEPLETED URANIUM HEXAFLUORIDE: WASTE OR RESOURCE? N. Schwertz, J. Zoller, R. Rosen, S. Patton LAWRENCE LIVERMORE NATIONAL LABORATORY P. -

Depleted Uranium Technical Brief
Disclaimer - For assistance accessing this document or additional information,please contact [email protected]. Depleted Uranium Technical Brief United States Office of Air and Radiation EPA-402-R-06-011 Environmental Protection Agency Washington, DC 20460 December 2006 Depleted Uranium Technical Brief EPA 402-R-06-011 December 2006 Project Officer Brian Littleton U.S. Environmental Protection Agency Office of Radiation and Indoor Air Radiation Protection Division ii iii FOREWARD The Depleted Uranium Technical Brief is designed to convey available information and knowledge about depleted uranium to EPA Remedial Project Managers, On-Scene Coordinators, contractors, and other Agency managers involved with the remediation of sites contaminated with this material. It addresses relative questions regarding the chemical and radiological health concerns involved with depleted uranium in the environment. This technical brief was developed to address the common misconception that depleted uranium represents only a radiological health hazard. It provides accepted data and references to additional sources for both the radiological and chemical characteristics, health risk as well as references for both the monitoring and measurement and applicable treatment techniques for depleted uranium. Please Note: This document has been changed from the original publication dated December 2006. This version corrects references in Appendix 1 that improperly identified the content of Appendix 3 and Appendix 4. The document also clarifies the content of Appendix 4. iv Acknowledgments This technical bulletin is based, in part, on an engineering bulletin that was prepared by the U.S. Environmental Protection Agency, Office of Radiation and Indoor Air (ORIA), with the assistance of Trinity Engineering Associates, Inc. -

ORGANOMETAT,T,TC CHEMISTRY of URANIUM a Thesis Submitted By
ORGANOMETAT,T,TC CHEMISTRY OF URANIUM A thesis submitted by R1TN R. SIGURDSON, B.Sc. for the DEGREE of DOCTOR of PHILOSOPHY of the UNIVERSITY of LONDON Royal College of Science Imperial College of Science and Technology London, SW7 ?AY August 1976 TO MY PARENTS 3 ACKNOWLEDGEMENTS I would like to express my gratitude to Professor Geoffrey Wilkinson, F.R.S. for his guidance and enthusiastic support throughout the course of this work. Many thanks are also extended to Drs. Dick Andersen, Ernesto Carmona-Guzman and David Cole-Hamilton for their suggestionS, encouragement and advice, and to Dr. Kostas Mertis for his patient help during the first months. I am indebted to the Canadian Research Council of Canada for financial support during the past three years. 4 CONTENTS ABSTRACT 6 INTRODUCTION I. The Chemistry of Uranium(IV) 8 .II. The Chemistry of Uranium(V) 15 III. The Chemistry of Uranium(VI) 16 CHAPTER I. DILITHIUMHEXAALKYLURANATE(IV) COMPLEXES I. Introduction 19 II. Results and Discussion 27 III. Experimental 35 CHAPTER II. TRILITHIUMOCTAALKYLURANATE(V) COMPLEXES I. Introduction 54 II. Results and Discussion 55 III. Experimental 60 CHAPTER III. ADDITION COMPOUNDS OF URANIUM(VI) HEXAISO-PROPDXIDE WITH LITHIUM, MAGNESIUM AND ALUMINIUM ALKYLS I. Introduction 70 II. Results and Discussion 71 III. Experimental 77 CHAPTER IV. ORGANOMETALLIC CHEMISTRY OF ADAMANTANE I. Introduction 84 II. Results and Discussion 85 III. Experimental 87 REFERENCES 92 5 ABBREVIATIONS Me - methyl Et - ethyl Prn- normal-propyl Pri- iso-propyl Bun- normal-butyl But- iso-butyl But- tertiary-butyl Ph - phenyl CP cyclopentadienyl DME - dimethoxyethane tmed - N,N,NI,N'-tetramethylethylenediamine pmdt - N,N,Nt,N",N"-pentamethyldiethylenetriamine g.l.c. -

Sources, Effects and Risks of Ionizing Radiation
SOURCES, EFFECTS AND RISKS OF IONIZING RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2016 Report to the General Assembly, with Scientific Annexes UNITED NATIONS New York, 2017 NOTE The report of the Committee without its annexes appears as Official Records of the General Assembly, Seventy-first Session, Supplement No. 46 and corrigendum (A/71/46 and Corr.1). The report reproduced here includes the corrections of the corrigendum. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, in most cases, those that were in use at the time the data were collected or the text prepared. In other cases, however, the names have been updated, where this was possible and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.17.IX.1 ISBN: 978-92-1-142316-7 eISBN: 978-92-1-060002-6 © United Nations, January 2017. All rights reserved, worldwide. This publication has not been formally edited. Information on uniform resource locators and links to Internet sites contained in the present publication are provided for the convenience of the reader and are correct at the time of issue. The United Nations takes no responsibility for the continued accuracy of that information or for the content of any external website. -

Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility
Journal of NUCLEAR SCIENCE and TECHNOLOGY, 8 〔9〕, p. 498~502 (September 1971). Preparation of Anhydrous Uranium Tetrachloride and Measurements on Its Magnetic Susceptibility Tetsuhiko YOSHIMURA*, Chie MIYAKE* and Shosuke IMOTO* Received May 24, 1971 The reaction of UO2 with CCl4 vapor was carried out in a vacuum of 10-5 mmHg at 500℃ in order to prepare UCl4 of high purity. The lattice parameters were calculated from X-ray analysis to be a0=8.278±0.002, c0=7.460±0.009. The product thus ohtained was verified by chemical and X-ray analyses to be of anhydride form. The magnetic susceptibility was measured over the temperature range from liquid N2 to liquid He temperature, which revealed a deviation from the straight 1/X-T relation toward lower temperature. and that the anhydrous compound had ortho- I. INTRODUCTION rhombic symmetry. Since the same morpho- In the U-Cl system, there are known to logy may also exist for the chloride also, the exist four compounds, UCl3, UCl4, UCl5 and UCl4 is required to be of high purity. UCl6. Their chemical, physical and thermo- Table 1 Physical properties of UCl4 dynamic properties have been extensively investigated(1)(2) as well as those of the fluo- rides. Among these compounds UCl4 is used as the starting material for the synthesis of various uranous complexes, for its high solubility in many organic polar solvents such as methanol, ethanol, acetone, as reported by Selbin et al. (3)~(5) and Bagnall et al. (6) This com- pound is also used as the starting material for the preparation of UC(7), UN, US2(1)(8),USe2(1) etc. -

Needs for Large Mass Phototype Corium Experiments
NEEDS FOR LARGE MASS PROTOTYPIC CORIUM EXPERIMENTS: THE PLINIUS-2 PLATFORM Christophe Journeau, Laurence Buffe, Jean François Haquet, Pascal Piluso, Guy Willermoz CEA DEN, Cadarache, SMTA, 13108 St Paul lès Durance, France [email protected]; [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Corium is the molten material formed after meltdown of a nuclear reactor core during a severe accident. In order to improve the understanding and modelling of corium behavior, experiments are needed both for LWRs and GenIV fast reactors. Experiments using low temperature simulant materials, thanks to lower costs and constraints, allow the testing of a larger number of configurations and the determination of correlations. But some crucial corium phenomena cannot be reproduced at low temperatures such as the importance of radiation heat transfer or the presence of a large (up to 1000 K) liquidus-solidus interval. Consequently, some experiments are performed with high temperature simulant materials: alumina thermite as well as refractory oxides. However, it is not feasible to simulate all the aspects of corium phenomenology, especially its high temperature physico-chemistry. Therefore, even though the use of depleted uranium implies a series of protective and regulatory measures, the need for prototypic corium experimented is supported through several examples Another important aspect of experiment design deals with scaling. Small or medium scale corium experiments are easier to operate and only a few large scale (>100 kg) facilities have been built. Several effects are only visible with significant masses, as for instance, the formation of a corium cake during FCI or all the phenomena controlled by crust strength, such as underwater spreading or corium jet ablation. -

System Studies of Fission-Fusion Hybrid Molten Salt Reactors
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2013 SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS Robert D. Woolley University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Nuclear Engineering Commons Recommended Citation Woolley, Robert D., "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2628 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Robert D. Woolley entitled "SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Nuclear Engineering. Laurence F. Miller, Major Professor We have read this dissertation and recommend its acceptance: Ronald E. Pevey, Arthur E. Ruggles, Robert M. Counce Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) SYSTEM STUDIES OF FISSION-FUSION HYBRID MOLTEN SALT REACTORS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Robert D. -

Export Control Handbook for Chemicals
Export Control Handbook for Chemicals -Dual-use control list -Common Military List -Explosives precursors -Syria restrictive list -Psychotropics and narcotics precursors ARNES-NOVAU, X 2019 EUR 29879 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Xavier Arnés-Novau Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-785421 Filippo Sevini Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-786793 EU Science Hub https://ec.europa.eu/jrc JRC 117839 EUR 29879 Print ISBN 978-92-76-11971-5 ISSN 1018-5593 doi:10.2760/844026 PDF ISBN 978-92-76-11970-8 ISSN 1831-9424 doi:10.2760/339232 Luxembourg: Publications Office of the European Union, 2019 © European Atomic Energy Community, 2019 The reuse policy of the European Commission is implemented by Commission Decision 2011/833/EU of 12 December 2011 on the reuse of Commission documents (OJ L 330, 14.12.2011, p. 39). Reuse is authorised, provided the source of the document is acknowledged and its original meaning or message is not distorted. The European Commission shall not be liable for any consequence stemming from the reuse. -

09 Fr0200361
09 FR0200361 ^r.^r IQ 34. IMPORTANCE OF PROTOTYPIC-CORIUM EXPERIMENTS FOR SEVERE ACCIDENT RESEARCH P. PILUSO, C. JOURNEAU, G. COGNET, D. MAGALLON*, J.M. SEILER** CEA Cadarache 13108 St Paul les Durance cedex, France *: detached from the Joint Research Centre of the European Commission ** CEA Grenoble KEY WORDS: Severe accidents, Nuclear materials, Experiments Introduction: In case of a severe accident in a nuclear reactor, very complex physical and chemical phenomena would occur. Parallel to the development of mechanistic and scenario codes, experiments are needed to determine key phenomena and coupling, develop and qualify specific models, validate codes. Due to lower costs and constraints, experiments using low temperature simulant materials (such as CORINE [1] or Scaled Simulant Spreading Experiments (S3C) [2] for spreading or BALI [3,4] for in-vessel pools) allow the testing of a large number of configurations and the determination of correlations. But some crucial corium phenomena and behaviours such as the importance of radiation heat transfer or the presence of a large liquidus-solidus interval (up to 1000 K) are not reproduced in low temperature experiments. Consequently, it is attempted to simulate real corium with high temperature simulant materials: alumina, thermite, widely used (e.g. KATS [5] or COMET [6] facilities); high temperature salts or mixtures with zirconia and/or hafnia. However, it is not feasible to simulate all the aspects of corium phenomenology, especially its high temperature behaviour. Therefore, experiments with prototypic material are performed to check the results obtained with simulants and identify possible differences. In this context, CEA has undertaken a large program on severe accidents with prototypic corium [7]. -
Jan. 15, 1952 C. D., WLDER 2,582,941 PROCESSES of PRODUCING URANIUM CHLORIDES Filed Oct
Jan. 15, 1952 C. D., WLDER 2,582,941 PROCESSES OF PRODUCING URANIUM CHLORIDES Filed Oct. 2, 1944 d S Q Q Q t 2. INVENTOR. 6ozEMAW Z. WZZZOER ATTORNEY . Patented Jan. 15, 1952 2,582,941 UNITED STATES PATENT OFFICE 2,582,941 PROCESSES OF PRODUCING URANUM CHELORDES - Coleman D. Wilder, Oak Ridge, Tenn., assignor to the United States. of America, as represented bysion the United States Atomic Energy Commis Application October 12, 1944, Serial No. 558,452 8 Claims. (C1. 23-145) 2. - This invention relates to the manufacture of a pating carbon tetrachloride vapor in a speedy uranium chloride product, and more particularly stream of dry air, passing the resultant mixture re of uranium pentachloride over an uranium compound heated to the neigh - - - - - a . g a substantial proportion of borhood of 550° C. whereby uranium pentachlo uranium hexachloride, by a process for chlorinat: ride is formed and vaporized into the current, ing various, compositions comprising uranium moving the resulting vaporous mixture into a compounds, with carbon tetrachloride vapor car collecting receptacle so that most of the uranium ried by a Swift current of air. chloride will condense and settle out, and send This invention has for an object the rapid pro ing the gas stream through a dust separator to duction of a uranium chloride product compris strip it of the residual uranium-containing par ing a large proportion of uranium pentachloride ticles. and various proportions of uranium hexachlo The apparatus employed comprises a device ride. for supplying carbon tetrachloride, a device for A further object of the invention is to provide supplying dry air, a flash boiler in which the a high-yield, low-loss process for the production 5 carbon tetrachloride is vaporized and mixed with of uranium pentachloride which is economical the air, a reaction chamber in which the ai and suitable for large scale production of said carbon tetrachloride admixture is reacted with chloride. -
![CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]](https://docslib.b-cdn.net/cover/9085/catalog-of-nuclear-reactor-concepts-disc-1-2699085.webp)
CATALOG of NUCLEAR REACTOR CONCEPTS [Disc 1]
ANL-7092 Reactor T e lcbnology (TID-4500, 46th Ed.) 1 AEC Research and D eve lopmerit Report ARGONNENATIONAL LABORATORY 9700 South Cass Avenue Argonne, Illinois 60440 CATALOG OF NUCLEAR REACTOR CONCEPTS Part I. Homogeneous and Quasi-homogeneous Reactors Section 111. Reactors Fueled withMolten- salt Solutions . Charles E. Teeter, James A. Lecky, and John H.Martens Technical Pu'blications Department September 1965 Operated by The University of Chicago da under i Contract W - 3 1 - 109-eng -38 with the U. S. Atomic Energy Commission 445b 0311q50 ' i21 a ! i 1 4 ? i 3 TABLE OF CONTENTS --Page Preface ........................................ 5 Plan of Catalog of Nuclear Reactor Concepts. ............... 6 SECTION 111. REACTORS FUELED WITH MOLTEN-SALT SOLUTIONS. ........................... 7 Chapter 1. Introduction. .......................... 7 Chapter 2. One-region Reactors .................... 11 Chapter 3. Two-region Reactors .................... !33 c 5 . - PREFACE This report is an additional section in the Catalog of Nuclear Reactor Concepts that was begun with ANL-6892 and continued in ANL-6909. As in the previous reports, the material is divided into chapters, each with text and references, plus data sheets that cover the individual concepts. The planof the catalog, with the report numbers for the sections already issued, is given on the following page. Dr. Charles E. Teeter, formerly employed by the Chicago Oper- . ations Office at Argonne, Illinois, is now affiliated with the Southeastern Massachusetts Technological Institute, New Bedford, Mass. Through. a consultantship arrangement with Argonne National Laboratory, he is con- c tinuing to help guide the organization and cofnpilation of this catalog. J.H.M. September, 1965 , PLANOFCATALOGOFREACTORCONCEPTS Gene r a1 Introduction ANL - 6 89 2 Part I.