Plant Vitamins Agronomic, Physiological, and Nutritional Aspects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agrumi Abc.Specie 2007
Tel: ++41 91/795 18 67 Fax:++41 91/795 30 29 e-mail: [email protected] internet: www.eisenhut.ch Agrumi abc.specie 2007 Nr. Atlantia 262 Citrus Citroides 105 Citrus aurantiifolia cv. Caribean Bears Lime Lime con frutto molto piccolo succoso, tipico Lime, pianta bella e 280 Citrus aurantiifolia cv. Itariet-ileg-lime SPS compatta* (C. aurantifolia x Fortunella japonica) - Ibridi tra Kumquat rotondo e limetta messicana. Crescita media ideale per tenere in vaso, ha una lunga fioritura. Frutto somigliante alla limetta Mexicana di color giallo, ma alla raccolta dovrebbe essere ancora verde, gusto non così amara come la limetta Mexicana.otiima limetta, non molto 133 Citrus aurantiifolia cv. Mexican Lime aspro, ne troppo amaro* cv. Mexico Limette CD Limetta acida Piccolo alberello, i giovani rametti sono provvisti di spine corte, rigide ed estremamente appuntite, foglie piccole, fiori piccoli, frutto piccolo, di forma ovale murità di color verde-giallo, buccia molto sottile, polpa bianco-verdastro. Originaria dell` arcipelago delle Indie orientali. Limetta mexicana succoso resta prraticamente verde l'unico, viene utilizzato per Teckila E` usata come sostituzione del limone e come pianta indicatrice del 143 Citrus aurantiifolia cv. Mexico Limette CD virus della tristezza, sensibile al freddo.* 283 Citrus aurantiifolia Limetta gialla 4 stagioni Frutto liscio e rotondo a maturazione precoce, senza spine 145 Citrus aurantiifolia 160 Citrus aurantium arancio amaro (non nr. 24) arancio amaro Arancio ornamentale molto amaro, se non subito raccolto divena secca solo 162 Citrus aurantium Rivera ornamentale* 373 Citrus aurantium Bizzaria Bouquet de Nice a fleurs 376 Citrus aurantium doubles C.R.C. -

Chimerism Evaluation of 'Hongrou Huyou', a Grafted Chimera Between
RESEARCH ARTICLE https://doi.org/10.7235/HORT.20200011 Chimerism Evaluation of ‘Hongrou Huyou’, a Grafted Chimera between Citrus changshan-huyou and Citrus unshiu Min Zhang1†*, Zequn Zhang1†, Qun Wu2, Fuzhi Ke3, Jianguo Xu3, Siqing Zhao4, Gang Wang4, and Chi Zhang1 1State Key Laboratory of Subtropical Silviculture, Zhejiang A& F University, Hangzhou 311300, PR China 2Quzhou Technical Extension Station for Cash Crops, Quzhou 324000, PR China 3Zhejiang Citrus Research Institute, Taizhou, Zhejiang 318020, PR China 4Changshan Huyou Research Institute, Quzhou, Zhejiang 324000, PR China *Corresponding author: [email protected] †These authors contributed equally to the work. Abstract Chimeras occur spontaneously or artificially and are valuable in horticultural crop breeding. A new diploid citrus chimera, named ‘Hongrou Huyou’ (abbreviated HH) (Citrus changshan-huyou + C. unshiu), was found during a bud sports investigation in China. The morphology, flesh carotenoid Received: April 30, 2019 content, and molecular markers were evaluated in HH and the two grafted donors. The results Revised: November 29, 2019 Accepted: December 8, 2019 showed that characteristics derived from L2/L3, such as the fruit size, winged leaf, seed, pollen, and rind aroma, were similar to those of C. changshan-huyou (CH), whereas the juice sac and stomatal characteristics originating from L1 were the same as those of the satsuma mandarin. High-performance OPEN ACCESS liquid chromatography analysis of carotenes from the flesh of HH showed that the content was the HORTICULTURAL SCIENCE and TECHNOLOGY same as that of the satsuma mandarin, with β-cryptoxanthin producing the main carotenoid spectrum, 38(1):107-117, 2020 whereas lutein and violaxanthin were the main carotenoids in CH. -

Citrus Nummerisch 2007
Tel: ++41 91/795 18 67 Fax:++41 91/795 30 29 e-mail: [email protected] internet: www.eisenhut.ch Citrus nummerisch 2007 Nr. Limone - bedornte Pflanze mittlerer Grösse mit grossen Blüten - eher kleinfrüchtig. Frucht essbar; dünne Schale; längliche Frucht mit saurem Fruchtfleisch. Der Saft der Frucht wird zu medizinischen 1 Citrus limon Zwecken verwendet. Arancio - molto buono 2 Citrus sinensis ZN Mandarino - Aussehen wie Mittelmeermandarine - in alter Liste als C. reticulata 3 Citrus reticulata Tangerino senza semi blanco bezeichnet. Frucht von Zitronengrösse, erinnert an eine Limonimedica, die stark 6 Citrus medica (?) genarbt ist und ein bis mehrere Höcker aufweist. 104/131/136/10/ 99 x Citrofortunella Floridana cv. Eustis Limequat (vermutlich eine Hybride zwischen C. limon und C. sinensis) 1908 durch F. N. Meyer aus China in die USA eingeführt - die Sorte sei 11 Citrus limon cv. Meyer kälteresistenter als alle übrigen Zitronen - gelborange bei Reife. panaschierte Zitrone mit gestreiften Früchten und weissem 12 Citrus limon cv. Limon variegata Fruchtfleisch Zitrone mit glatte Schale, eher kleinfrüchtig, Geschmack wie Meyer- 13 Citrus limon cv. Buccia liscia Zitrone. 14 Citrus limon cv. Calabria 15 Citrus limon cv. Dolce Aussehen wie kleine Zitronen - süss, etwas fade im Geschmack. cv. Quattro stagioni "Vier-Jahreszeiten-Zitrone" - starkwachsend - sie trägt zu allen vier 16 Citrus limon (=Lunario) Jahreszeiten gelcihzeitig Blüten und Früchte. 17 Citrus limon cv. Albergo Camelia Zitrone aus der Albergo Camelia - kleinfrüchtig. 18 Citrus limon cv. Scob Round runde und grosse Früchte 19 Citrus limon cv. Primo fiore frühreife Sorte, frühblühend, wenige aber äusserst grosse Früchte. (teilweise mit kleinen Töchterfrüchten) Blätter stark duftend - aus den Blättern wird Duftöl gewonnen. -
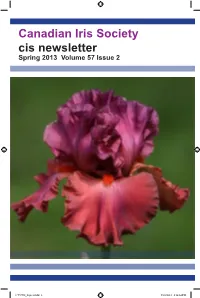
Canadian Iris Society Cis Newsletter Spring 2013 Volume 57 Issue 2
Canadian Iris Society cis newsletter Spring 2013 Volume 57 Issue 2 C-V57N2_layout.indd 1 5/28/2013 2:14:54 PM Canadian Iris Society Board of Directors Officers for 2013 President Ed Jowett, 1960 Sideroad 15, RR#2 Tottenham, ON L0G 1W0 2014-2016 ph: 905-936-9941 email: [email protected] 1st Vice John Moons, 34 Langford Rd., RR#1 Brantford ON N3T 5L4 2014-2016 President ph: 519-752-9756 2nd Vice Harold Crawford, 81 Marksam Road, Guelph, ON N1H 6T1 (Honorary) President ph: 519-822-5886 e-mail: [email protected] Secretary Nancy Kennedy, 221 Grand River St., Paris, ON N3L 2N4 2014-2016 ph: 519-442-2047 email: [email protected] Treasurer Bob Granatier, 3674 Indian Trail, RR#8 Brantford ON N3T 5M1 2014-2016 ph: 519-647-9746 email: [email protected] Membership Chris Hollinshead, 3070 Windwood Dr, Mississauga, ON L5N 2K3 2014-2016 ph: 905 567-8545 e-mail: [email protected] Directors at Large Director Gloria McMillen, RR#1 Norwich, ON N0J 1P0 2011-2013 ph: 519 468-3279 e-mail: [email protected] Director Ann Granatier, 3674 Indian Trail, RR#8 Brantford ON N3T 5M1 2013-2015 ph: 519-647-9746 email: [email protected] Director Alan McMurtrie, 22 Calderon Cres. Wlllowdale ON M2R 2E5 2013-2015 ph: 416-221-4344 email: [email protected] Director Pat Loy 18 Smithfield Drive, Etobicoke On M8Y 3M2 2013-2015 ph: 416-251-9136 email: [email protected] Honorary Director Hon. Director David Schmidt, 18 Fleming Ave., Dundas, ON L9H 5Z4 Webmaster Chris Hollinshead, 3070 Windwood Dr, Mississauga, ON L5N 2K3 ph: 905 567-8545 e-mail: [email protected] Newsletter Ed Jowett, 1960 Sideroad 15, RR#2 Tottenham, ON L0G 1W0 Editor ph: 905-936-9941 email: [email protected] Newsletter Vaughn Dragland Designer ph. -

D E L L E P I a S T E C Ii E Si C O L T I V A
DELLE PIASTE C II E SI COLTIVANO s \ 5133 l R. ORTO BOTANICO DI NAPOLI CORREDATO DELLA PIANTA DEL MEDESIMO , E DI ANNOTAZIONI. NAPOLI TIPOGRAFIA DELL’ AQUILA DI V. PUZZIELLO Nei Chiostro S. Tommaso d’ Aquino. 1 8 4 5 , i i m i t i l i ! ! Diverse notizie trovandosi date fuori intorno al nostro Reai Orto Botanico, non sarà me stieri farne il soggetto di altro apposito ragionare. Tuttavia in grazia di coloro che lette non le abbiano nel D iscorso per me dettato all’ occasione della solenne apertura della scuola an nessa a questo Stabilimento (i) ; negli Annali Civili del Regno (a) , od in altre più recen ti pubblicazioni, gioverà rammentare come al primo cominciamenlo dell’ Orto attuale fosse data opera nel 1809 , col trasferirvi le poche piante riunite in un orticello che , a premu ra del mio predecessore Cav. Vincenzio Pelagna erasi introdotto nel giardino di Monte Olive- to , addetto poscia a mercato di commestibili. Prima di quel tempo un vero Orto botanico presso noi non esisteva , e risalir conviene fino al 1662 per rinvenirne qualche vestigio nel- 1’ Orio de' semplici della Montagnola , piantato a cura del governo della pia casa della SS. Annunziata. Non mancavano, egli è vero, prima e dopo di quel tempo distinte persone invaghite della coltura delle più rare e pregevoli piante ; che perciò famosi se ne rendevano in epoche più rirnote gli Orti del P in e lli e del P o r la , ed in tempi a noi più vicini quelli dei S a n s e v e n n i, de’ d r i l l i e de’ P o li ; non che le importanti collezioni di piante esotiche intro dotte nel R. -

Grapefruit: History, Use, and Breeding Portant Agricultural Commodity
Barbados to its development as an im- Grapefruit: History, Use, and Breeding portant agricultural commodity. We review the progress and fluctuations Eliezer S. Louzada1 and Chandrika Ramadugu2 in production and market aspects. Be- cause grapefruit is valued as a highly nutritious fruit, we will discuss the sig- ADDITIONAL INDEX WORDS. biotechnology, drug interaction, mutation breeding, nificant health benefits associated with natural mutation, protoplast, somatic hybrids it. Interaction of grapefruit with cer- SUMMARY. Grapefruit [Citrus 3aurantium (synonym C. 3paradisi)] is an tain commonly used drugs has been important citrus commodity that originated in Barbados in the 17th century. reported; we examine the mode of in- Citrus Grapefruit is the youngest member of the genus . Most commercially teractions and address this concern. important grapefruit cultivars arose through natural and induced mutations, not The first commercial cultivation traditional breeding, of the white-fleshed and seedy Duncan grapefruit. Now, cultivars with a range of flesh colors exist; the pigmentation is correlated with of grapefruit occurred less than 200 lycopene content. A bud sport mutant of grapefruit discovered in Texas has a years ago. The original grapefruit tree deep golden-colored flesh, significantly different from the typical reddish produced very seedy fruit with white pigmentation. In this review, we discuss grapefruit’s journey from its origin in flesh; however, today, several different Barbados and its global establishment including production, marketing, -

Hyperacidification of Citrus Fruits by a Vacuolar Proton-Pumping P-Atpase Complex
UvA-DARE (Digital Academic Repository) Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex Strazzer, P.; Spelt, C.E.; Li, S.; Bliek, M.; Federici, C.T.; Roose, M.L.; Koes, R.; Quattrocchio, F.M. DOI 10.1038/s41467-019-08516-3 Publication date 2019 Document Version Final published version Published in Nature Communications License CC BY Link to publication Citation for published version (APA): Strazzer, P., Spelt, C. E., Li, S., Bliek, M., Federici, C. T., Roose, M. L., Koes, R., & Quattrocchio, F. M. (2019). Hyperacidification of Citrus fruits by a vacuolar proton-pumping P- ATPase complex. Nature Communications, 10, [744]. https://doi.org/10.1038/s41467-019- 08516-3 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:02 Oct 2021 ARTICLE https://doi.org/10.1038/s41467-019-08516-3 OPEN Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex Pamela Strazzer 1, Cornelis E. -

Rodin Et Les Oranges Bleues
Rodin et les oranges bleues Autor(en): Zbinden, Véronique Objekttyp: Article Zeitschrift: Générations Band (Jahr): - (2016) Heft 85 PDF erstellt am: 02.10.2021 Persistenter Link: http://doi.org/10.5169/seals-830742 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch PASSION PASSION Rodin et les LE CALAMONDIN (CITRUS MITIS) Le seul agrume qui se porte bien bleues à l'intérieur. Les pépiniéristes l'ont oranges rebaptisé «calamansi» et décrété qu'il était décoratif. Faux. Originaire des Philippines, il est utilisé Lointain descendant d'Auguste, l'immense sculpteur, là-bas vert dans des plats cuisinés ou des cocktails. -
Musei Scientifici a Firenze
Musei Scientifici a Firenze Florence Science Museums Indice/Index APT Firenze Map 4 Via A. Manzoni 16 Mappa ............................................................................................................ 5 50121 FIRENZE tel. 05523320 fax 0552346286 www.firenzeturismo.it Preface by Margherita Hack 6 Presentazione di Margherita Hack ................................................. 7 Introduction Science in Florence 10 Introduzione Firenze e la scienza ................................................. 11 Must-see attractions 14 Da non perdere ........................................................................................15 Testi a cura di/text by Giardino di Archimede, Museo di Storia della Scienza, Fondazione Scienza e Tecnica, Museo di Storia Naturale The Garden of Archimedes. A Museum for Mathematics 16 Il Giardino di Archimede. Un Museo per la Matematica ......17 Si ringraziano per la collaborazione prof. Enrico Giusti, prof. Paolo Galluzzi, dott. Guido Gori e prof. Giovanni Pratesi. Science and Technics Foundation. Planetarium 22 Fondazione Scienza e Tecnica. Planetario ................................23 Coordinamento redazionale/editorial management APT Firenze in collaborazione con i singoli musei Museum of the History of Science 30 Crediti fotografici/photo credits Museo di Storia della Scienza ..........................................................31 Museo di Storia della Scienza – Franca Principe; Archivio fotografico Fondazione Scienza e Tecnica; Museo di Storia Naturale – Saulo Bambi, Angelo Faiazza, Andrea -

The Far-Reaching Contributions of Bud Sport Mutants to Horticulture and Plant Biology Toshi M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by IRTA Pubpro Foster and Aranzana Horticulture Research (2018) 5:44 Horticulture Research DOI 10.1038/s41438-018-0062-x www.nature.com/hortres REVIEW ARTICLE Open Access Attention sports fans! The far-reaching contributions of bud sport mutants to horticulture and plant biology Toshi M. Foster 1 and Maria José Aranzana2,3 Abstract A bud sport is a lateral shoot, inflorescence or single flower/fruit with a visibly different phenotype from the rest of the plant. The new phenotype is often caused by a stable somatic mutation in a single cell that is passed on to its clonal descendants and eventually populates part or all of a meristem. In many cases, a bud sport can be vegetatively propagated, thereby preserving the novel phenotype without sexual reproduction. Bud sports provide new characteristics while retaining the desirable qualities of the parent plant, which is why many bud sports have been developed into popular cultivars. We present an overview of the history of bud sports, the causes and methods of detecting somaclonal variation, and the types of mutant phenotypes that have arisen spontaneously. We focus on examples where the molecular or cytological changes causing the phenotype have been identified. Analysis of these sports has provided valuable insight into developmental processes, gene function and regulation, and in some cases has revealed new information about layer-specific roles of some genes. Examination of the molecular changes causing a phenotype and in some cases reversion back to the original state has contributed to our understanding of the mechanisms that drive genomic evolution. -

WO 2017/151835 Al 8 September 2017 (08.09.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/151835 Al 8 September 2017 (08.09.2017) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/34 (2006.01) A61K 8/98 (2006.01) kind of national protection available): AE, AG, AL, AM, A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 17/0203 12 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, (22) International Filing Date: KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, 2 March 2017 (02.03.2017) MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, (25) Filing Language: English RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, (26) Publication Language: English TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 62/302,972 3 March 2016 (03.03.2016) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: ACCESS BUSINESS GROUP INTERNA¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TIONAL LLC [US/US]; 7575 Fulton Street East, Grand TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, Rapids, Michigan 49355 (US). -

Michael Ceron, "Der Zitrusgarten" Blumenweg 3 9583 Faak Am See Mitgliedsnummer K-1942 Betriebsnummer 0500089
2018 Ihr Betrieb wurde im Auftrag von BIO – AUSTRIA von der Bio-Kontrollstelle LACON GmbH (AT-BIO-402), 4150 Rohrbach, Am Teich 2 kontrolliert und erfüllt den „BIO AUSTRIA Standard“. Dieses Zertifikat ist gültig in Verbindung mit dem Zertifikat der Bio-Kontrollstelle gemäß Verordnung (EG) 834/2007 und umfasst • die Pflanzenbauerzeugnisse (Ernte 2018) Michael Ceron, "Der Zitrusgarten" Blumenweg 3 9583 Faak am See Mitgliedsnummer K-1942 Betriebsnummer 0500089 Ihr Betrieb ist berechtigt, die auf dem gültigen Zertifikat der Bio-Kontrollstelle angeführten Bio-Produkte und die Bio-Umstellungsprodukte unter Einhaltung der Deklarationsvorschriften der Verordnung (EG) 834/2007 mit dem BIO-AUSTRIA Warenhinweis zu vertreiben. Basis für die Aufrechterhaltung der Gültigkeit des Zertifikats: - aufrechte Mitgliedschaft oder Zertifizierungsvereinbarung mit BIO AUSTRIA - aufrechtes Kontrollverhältnis mit der Bio-Kontrollstelle Ausgestellt am 27.12.2018 Für die Kontrollstelle LACON Für BIO AUSTRIA Ing. Erwin Huber, MA, Geschäftsführer Gerti Grabmann, Obfrau BIOLOGISCHE ERZEUGUNG 2018 Dem Unternehmen auszustellende Bescheinigung gemäß Artikel 29, Absatz 1 der Verordnung (EG) Nr. 834/2007 Michael Ceron, "Der Zitrusgarten" 9583 Faak am See, Blumenweg 3 Betriebsnummer 0500089 Haupttätigkeit: Erzeugung / Verarbeitung AT-BIO-402 Pflanzen und pflanzliche Erzeugnisse Gültigkeitsdauer von 27.12.2018 bis 31.01.2020 ⁽¹⁾ Biologisch: Zitrusjungpflanzen: C. sinensis Tarocco, C. limon Florentina, C. limon Variegatum, C. sinensis Oblungus, C. limon Lunario, C. Keraji, C. sinensis Navel, C. limon Bizzaro, C. limon Amalphitanum Ornamentale, C. medica Digitata, C. medica Maxima, C. aurantifolia La Valetta, C. limetta Pursha, Calamondien, C. otaitensis, C. Limquat, C. paradisi Royal Gelb, C. deliciosa x C. paradisi, C. aurantifolia Philippine Red Lime, C. paradisi Ruby Red, C. aurantium x C.