Engineering Thermodynamics Summary of Topics from University of Washington Course ME323: Engineering Thermodynamics Taught Winter 2016 by Prof
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -
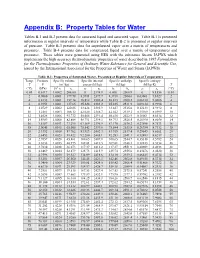
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -
![[5] Magnetic Densimetry: Partial Specific Volume and Other Applications](https://docslib.b-cdn.net/cover/6742/5-magnetic-densimetry-partial-specific-volume-and-other-applications-566742.webp)
[5] Magnetic Densimetry: Partial Specific Volume and Other Applications
74 MOLECULAR WEIGHT DETERMINATIONS [5] [5] Magnetic Densimetry: Partial Specific Volume and Other Applications By D. W. KUPKE and J. W. BEAMS This chapter consists of three parts. Part I is a summary of the principles and current development of the magnetic densimeter, with which density values may be obtained conveniently on small volumes of solution with the speed and accuracy required for present-day ap- plications in protein chemistry. In Part II, the applications, including definitions, are outlined whereby the density property may be utilized for the study of protein solutions. Finally, in Part III, some practical aspects on the routine determination of densities of protein solutions by magnetic densimetry are listed. Part I. Magnetic Densimeter The magnetic densimeter measures by electromagnetic methods the vertical (up or down) force on a totally immersed buoy. From these measured forces together with the known mass and volume of the buoy, the density of the solution can be obtained by employing Archimedes' principle. In practice, it is convenient to eliminate evaluation of the mass and volume of the buoy and to determine the relation of the mag- netic to the mechanical forces on the buoy by direct calibration of the latter when it is immersed in liquids of known density. Several workers have devised magnetic float methods for determin- ing densities, but the method first described by Lamb and Lee I and later improved by MacInnes, Dayhoff, and Ray: is perhaps the most accurate means (~1 part in 106) devised up to that time for determining the densities of solutions. The magnetic method was not widely used, first because it was tedious and required considerable manipulative skill; second, the buoy or float was never stationary for periods long enough to allow ruling out of wall effects, viscosity perturbations, etc.; third, the technique required comparatively large volumes of the solution (350 ml) for accurate measurements. -

Ventricular Rhythms Originate Below the Branching Portion of The
Northwest Community Healthcare Paramedic Program VENTRICULAR DYSRHYTHMIAS Pacemakers Connie J. Mattera, M.S., R.N., EMT-P Reading assignments: Bledsoe Vol 3; pp. 96-109 SOPs: VT with pulse; Ventricular fibrillation/PVT; Asystole/PEA Drugs: Amiodarone, magnesium, epinephrine 1mg/10mL Procedure manual: Defibrillation; Mechanical Circulatory Support (MCS) using a Ventricular Assist Device KNOWLEDGE OBJECTIVES: Upon completion of the reading assignments, class and homework questions, reviewing the SOPs, and working with their small group, each participant will independently do the following with at least an 80% degree of accuracy and no critical errors: 1. Identify the intrinsic rates, morphology, conduction pathways, and common ECG features of ventricular beats/rhythms. 2. Identify on a 6-second strip the following: a) Idioventricular rhythm b) Accelerated idioventricular rhythm c) Ventricular tachycardia: monomorphic & polymorphic d) Ventricular escape beats e) Premature Ventricular Contractions (PVCs) f) Ventricular fibrillation g) Asystole h) Paced rhythms i) Intraventricular conduction defects (Bundle branch blocks) 3. Systematically evaluate each complex/rhythm for the following: a) Rate (atrial and ventricular) b) Rhythm: Regular/irregular - R-R Interval, P-P Interval c) Presence/absence/morphology of P waves d) Presence/absence/morphology of QRS complexes d) P-QRS relationships f) QRS duration 4. Correlate the cardiac rhythm with patient assessment findings to determine the emergency treatment for each rhythm according to NWC EMSS SOPs. 5. Discuss the action, prehospital indications, side effects, dose and contraindications of the following during VT a) Amiodarone b) Magnesium 6. Describe the indications, equipment needed, critical steps, and patient monitoring parameters for cardioversion and defibrillation. 7. Identify the management of a patient with an implanted defibrillator and/or pacemaker. -

The Aircraft Propulsion the Aircraft Propulsion
THE AIRCRAFT PROPULSION Aircraft propulsion Contact: Ing. Miroslav Šplíchal, Ph.D. [email protected] Office: A1/0427 Aircraft propulsion Organization of the course Topics of the lectures: 1. History of AE, basic of thermodynamic of heat engines, 2-stroke and 4-stroke cycle 2. Basic parameters of piston engines, types of piston engines 3. Design of piston engines, crank mechanism, 4. Design of piston engines - auxiliary systems of piston engines, 5. Performance characteristics increase performance, propeller. 6. Turbine engines, introduction, input system, centrifugal compressor. 7. Turbine engines - axial compressor, combustion chamber. 8. Turbine engines – turbine, nozzles. 9. Turbine engines - increasing performance, construction of gas turbine engines, 10. Turbine engines - auxiliary systems, fuel-control system. 11. Turboprop engines, gearboxes, performance. 12. Maintenance of turbine engines 13. Ramjet engines and Rocket engines Aircraft propulsion Organization of the course Topics of the seminars: 1. Basic parameters of piston engine + presentation (1-7)- 3.10.2017 2. Parameters of centrifugal flow compressor + presentation(8-14) - 17.10.2017 3. Loading of turbine blade + presentation (15-21)- 31.10.2017 4. Jet engine cycle + presentation (22-28) - 14.11.2017 5. Presentation alternative date Seminar work: Aircraft engines presentation A short PowerPoint presentation, aprox. 10 minutes long. Content of presentation: - a brief history of the engine - the main innovation introduced by engine - engine drawing / cross-section - -

Carnot Cycles
CARNOT CYCLES Sadi Carnot was a French physicist who proposed an “ideal” cycle for a heat engine in 1824. Historical note – the idea of an ideal cycle came about because engineers were trying to develop a steam engine (a type of heat engine) where they could reject (waste) a minimal amount of heat. This would produce the best efficiency since η = 1 – (QL/QH). Carnot proposed that a cycle comprised of completely (internally and externally) reversible processes would give the maximum amount of net work for a given heat input, since the work done by a system in a reversible (ideal) process is always greater than that in an irreversible (real) process. THE CARNOT HEAT ENGINE CYCLE CONSISTS OF FOUR REVERSIBLE PROCESSES IN A SEQUENCE: 1 Æ 2: Reversible isothermal expansion. Heat transfer from HTR (+) and boundary work (+) occur in closed system 2 Æ 3: Reversible adiabatic expansion Work output (+), but no heat transfer 3 Æ 4: Reversible isothermal compression Heat transfer (-) and boundary work (-) occur in closed system 4 Æ 1: Reversible adiabatic compression Work input (-), but no heat transfer AND Wout >>> Win 1 P-V DIAGRAM FOR CARNOT HEAT ENGINE CYCLE P 1 2 4 3 Showing net work is POSITIVE. V A useful example of an isothermal expansion is boiling (vaporization) at a constant pressure in a device such as a piston-cylinder. Similarly, an example of an isothermal compression is condensation at a constant pressure in a piston-cylinder. Also, heat transfer can only occur in processes 1 Æ 2 and 3 Æ4. 1 Æ 2: since work is positive (expansion) and Δu is positive (e.g., boiling) then heat transfer is positive (input from HTR). -

Real Gases – As Opposed to a Perfect Or Ideal Gas – Exhibit Properties That Cannot Be Explained Entirely Using the Ideal Gas Law
Basic principle II Second class Dr. Arkan Jasim Hadi 1. Real gas Real gases – as opposed to a perfect or ideal gas – exhibit properties that cannot be explained entirely using the ideal gas law. To understand the behavior of real gases, the following must be taken into account: compressibility effects; variable specific heat capacity; van der Waals forces; non-equilibrium thermodynamic effects; Issues with molecular dissociation and elementary reactions with variable composition. Critical state and Reduced conditions Critical point: The point at highest temp. (Tc) and Pressure (Pc) at which a pure chemical species can exist in vapour/liquid equilibrium. The point critical is the point at which the liquid and vapour phases are not distinguishable; because of the liquid and vapour having same properties. Reduced properties of a fluid are a set of state variables normalized by the fluid's state properties at its critical point. These dimensionless thermodynamic coordinates, taken together with a substance's compressibility factor, provide the basis for the simplest form of the theorem of corresponding states The reduced pressure is defined as its actual pressure divided by its critical pressure : The reduced temperature of a fluid is its actual temperature, divided by its critical temperature: The reduced specific volume ") of a fluid is computed from the ideal gas law at the substance's critical pressure and temperature: This property is useful when the specific volume and either temperature or pressure are known, in which case the missing third property can be computed directly. 1 Basic principle II Second class Dr. Arkan Jasim Hadi In Kay's method, pseudocritical values for mixtures of gases are calculated on the assumption that each component in the mixture contributes to the pseudocritical value in the same proportion as the mol fraction of that component in the gas. -

Lecture 10. Heat Engines (Ch. 4)
Lecture 11. Heat Engines (Ch. 4) A heat engine – any device that is capable of converting thermal energy (heating) into mechanical energy (work). We will consider an important class of such devices whose operation is cyclic. Heating – the transfer of energy to a system by thermal contact with a reservoir. Work – the transfer of energy to a system by a change in the external parameters (V, el.-mag. and grav. fields, etc.). The main question we want to address: what are the limitations imposed by thermodynamic on the performance of heat engines? Perpetual Motion Machines are Impossible Perpetual Motion Machines of the first type – these designs seek to violation of the First Law create the energy required for their (energy conservation) operation out of nothing. Perpetual Motion Machines of the second type - these designs extract the energy required for their operation violation of the Second in a manner that decreases the entropy Law of an isolated system. hot reservoir Word of caution: for non-cyclic processes, T H 100% of heat can be transformed into work without violating the Second Law. heat Example: an ideal gas expands isothermally work being in thermal contact with a hot reservoir. Since U = const at T = const, all heat has been transformed into work. impossible cyclic heat engine Fundamental Difference between Heating and Work - is the difference in the entropy transfer! Transferring purely mechanical energy to or from a system does not (necessarily) change its entropy: ΔS = 0 for reversible processes. For this reason, all forms of work are thermodynamically equivalent to each other - they are freely convertible into each other and, in particular, into mechanical work. -

A Simplified and Structured Teaching Tool for the Evaluation and Management of Pulseless Electrical Activity
R e v i e w Med Princ Pract 2014;23:1–6 Received: February 27, 2013 DOI: 10.1159/000354195 Accepted: July 2, 2013 Published online: August 13, 2013 A Simplified and Structured Teaching Tool for the Evaluation and Management of Pulseless Electrical Activity a b a, c L a s z l o L i t t m a n n Devin J. Bustin Michael W. Haley a b c D e p a r t m e n t s o f Internal Medicine and Emergency Medicine, and Pulmonary and Critical Care Consultants, Carolinas Medical Center, Charlotte, N.C., USA K e y W o r d s Introduction Pulseless electrical activity · Cardiopulmonary resuscitation · Electrocardiogram · Echocardiogram Patients with pulseless electrical activity (PEA) ac- count for up to 30% of cardiac arrest victims [1, 2] . The survival rate of patients with PEA is much worse than that A b s t r a c t of cardiac arrest patients with shockable rhythms [1, 3] . Cardiac arrest victims who present with pulseless electrical Studies suggest that cause-specific treatment of PEA is activity (PEA) usually have a grave prognosis. Several condi- more effective than general treatments offered by ad- tions, however, have cause-specific treatments which, if ap- vanced cardiac life support (ACLS) guidelines such as plied immediately, can lead to quick and sustained recovery. cardiac massage, epinephrine and vasopressin [4] . High- Current teaching focuses on recollection of numerous con- er-dose epinephrine has actually been shown to be associ- ditions that start with the letters H or T as potential causes of ated with worse outcomes [5] . -

Real Gas Behavior Pideal Videal = Nrt Holds for Ideal
Real gas behavior Pideal Videal = nRT holds for ideal gas behavior. Ideal gas behavior is based on KMT, p. 345: 1. Size of particles is small compared to the distances between particles. (The volume of the particles themselves is ignored) So a real gas actually has a larger volume than an ideal gas. Vreal = Videal + nb n = number (in moles) of particles Videal = Vreal - nb b = van der Waals constant b (Table 10.5) Pideal (Vreal - nb) = nRT But this only matters for gases in a small volume and with a high pressure because gas molecules are crowded and bumping into each other. CHEM 102 Winter 2011 Ideal gas behavior is based on KMT, p. 345: 3. Particles do not interact, except during collisions. Fig. 10.16, p. 365 Particles in reality attract each other due to dispersion (London) forces. They grab at or tug on each other and soften the impact of collisions. So a real gas actually has a smaller pressure than an ideal gas. 2 Preal = Pideal - n a n = number (in moles) 2 V real of particles 2 Pideal = Preal + n a a = van der Waals 2 V real constant a (Table 10.5) 2 2 (Preal + n a/V real) (Vreal - nb) = nRT Again, only matters for gases in a small volume and with a high pressure because gas molecules are crowded and bumping into each other. 2 CHEM 102 Winter 2011 Gas density and molar masses We can re-arrange the ideal gas law and use it to determine the density of a gas, based on the physical properties of the gas.