Fermentation Results and Chemical Composition of Agricultural
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Practical Handbook on the Distillation of Alcohol from Farm
•^ '' .:,. .^t A PRACTICAL HANDBOOK ON THE Distillation of alcohol FROM FARM PRODUCTS INCLUDING The Processes of Malting; Mashing and Mascerating; Ferment- ing and Distilling Alcohol from Grain, Beets, Potatoes, Molasses, etc., with Chapters on Alcoholometry and the DE-NATURING OF ALCOHOL FOR USE IN Farm Engines, Automobiles, Launch Motors, and in Heating and Lighting; with a Synopsis of the New Free Alcohol Law and its Amendment and the Government Regulations. BY F. B. WRIGHT. SECOND EDITION. REVISED AND GREATLY ENLARGED NEW YORK SPON & CHAMBERLAIN, 120 Liberty Street LONDON E. & F. N. SPON. Limited. 57 Haymarket, S.W. 1918 >'>f/: x/'Av^-;.:,^ Copyright. 1906, By SPON & CHAMBERLAIN. Copyright, 1907. By SPON & CHAMBERLAIN. CAUBLOT PRESS. £26 William 8tr««t. Naw York. U. 8. A. PREFACE TO SECOND EDITION. " Since the passage of the " Free Alcohol Act there has been a constantly increasing demand for information as to the manufacture of industrial alcohol. This, with the favorable reception ac- corded to the first edition of this book has lead the publishers to bring out a second edition. The entire volume has been carefully revised and not only has the original text been amplified but new chapters have been added explaining the most modem and approved methods and appliances both as used in Europe and in this country. An- other valuable feature of the present volume is the collection of U. S. de-naturing formulas covering the special denaturants necessitated by the various arts and by the Government requirements. The chapters on modem distilling apparatus rectifiers and modem plants have been very carefully pre- pared in order to give the reader a clear idea of the various types of apparatus in use to-day and of their general place in a distillery system. -

The Perceptual Categorisation of Blended and Single Malt Scotch Whiskies Barry C
Smith et al. Flavour (2017) 6:5 DOI 10.1186/s13411-017-0056-x RESEARCH Open Access The perceptual categorisation of blended and single malt Scotch whiskies Barry C. Smith1*, Carole Sester2, Jordi Ballester3 and Ophelia Deroy1 Abstract Background: Although most Scotch whisky is blended from different casks, a firm distinction exists in the minds of consumers and in the marketing of Scotch between single malts and blended whiskies. Consumers are offered cultural, geographical and production reasons to treat Scotch whiskies as falling into the categories of blends and single malts. There are differences in the composition, method of distillation and origin of the two kinds of bottled spirits. But does this category distinction correspond to a perceptual difference detectable by whisky drinkers? Do experts and novices show differences in their perceptual sensitivities to the distinction between blends and single malts? To test the sensory basis of this distinction, we conducted a series of blind tasting experiments in three countries with different levels of familiarity with the blends versus single malts distinction (the UK, the USA and France). In each country, expert and novice participants had to perform a free sorting task on nine whiskies (four blends, four single malts, one single grain, plus one repeat) first by olfaction, then by tasting. Results: Overall, no reliable perceptual distinction was revealed in the tasting condition between blends and single malts by experts or novices when asked to group whiskies according to their similarities and differences. There was nonetheless a clear effect of expertise, with experts showing a more reliable classification of the repeat sample. -

Brewing Grains What Is Malt?
612.724.4514 [email protected] www.aperfectpint.net Brewing Grains Brewing grains are the heart and soul of beer. Next to water they make up the bulk of brewing ingredients. Brewing grains provide the sugars that yeast ferment. They are the primary source of beer color and a major contributor to beer flavor, aroma, and body. Proteins in the grains give structure to beer foam and minerals deliver many of the nutrients essential to yeast growth. By far the most common brewing grain is malted barley or barley malt, but a variety of other grains, both malted and unmalted, are also used including wheat, corn, rice, rye, and oats. What is Malt? To put it plainly, malt is cereal grain that has undergone the malting process. In the simplest terms, malting is the controlled germination and kilning of grain. Malting develops the diastatic enzymes that accomplish the conversion of starch to sugar during brewing and begins a limited process of conversion that makes the starches more accessible to the brewer. Malting also gives brewing grains their distinctive colors and flavors. Only the highest quality grain, called brewing grade, is selected for malting. Brewing grade grain is selected for, among other things, high starch content, uniform kernel size, low nitrogen content, and high diastatic power. Diastatic power is the ability of grains to break down complex starch molecules into simpler sugars for brewing. It is determined by the amount of diastatic enzymes in the grain. Barley is the most commonly malted grain, but other grains like wheat and rye are also malted. -

COCKTAIL & BAR LIST Champagne and Wines
COCKTAIL & BAR LIST Champagne and Wines Please ask your waiter to see the full wine list Champagne and Sparkling Glass Bottle Prosecco IGT Veneto, Casa Botter 7.50 28.00 Marie Demets NV 10.00 52.00 Le Mesnil Grand Cru Rose Brut NV 11.50 55.00 Bérèche & Fils NV Brut, Réserve 55.00 Perrier Jouet Grand Brut 60.00 Perrier Jouet Blason Rosé Champagne 85.00 Dom Perignon Vintage 2003 195.00 Krug Grande Cuvée 200.00 White Wine Glass Carafe 500ml Bottle Fontareche 2014 Vin de Pays D'Oc 5.00 15.00 19.00 Viognier 2014 Domaine de Coudoulet 6.50 19.50 24.00 Pinot Grigio Il Palu 2014, Venezia 7.00 21.00 27.00 Sauvignon Blanc, Jules Taylor 2014 NZ 8.50 26.00 33.00 Saint Véran, 2014 Domaine Michel Chavet 8.50 26.00 34.00 Rose Wine Glass Carafe 500ml Bottle Chateau Fontareche 2014 Corbieres 6.00 18.00 24.50 Red Wine Glass Carafe 500ml Bottle Domaine Felines-Jourdan 2014 Grenache/Syrah 5.00 15.00 19.00 Monte Alentejano 2010 Portugal 5.75 18.00 22.50 Chateau des Antonins 2012 Bordeaux Superieur 7.00 21.00 27.00 Côtes du Rhône 2013 Domaine Saint Gayan 7.50 22.50 30.00 Bourgogne Rouge, 2013 Le Clos, Manciat-Poncet 9.50 28.50 38.00 Some of our drinks may contain Allergens. If you have any allergies or Health Concerns Please notify a member of staff before ordering Beer VODKA Tyskie - 33cl - Lager, 5.6% 3.75 Pszeniczniak - 50cl - Wheat; 5,25% 5.50 Ognisko Flavours Light Lager A classic cloudy Wheat Beer Made with Fresh Fruit, Herbs and Spices Shot £3.75, Carafes 10cl - £14.50, 25cl - £35.00, 50cl- £68.00 Koź lak - 50cl - Dark Bock; 6,6% 5.80 Żywe - 50cl - Lager; -

The Alcohol Textbook 4Th Edition
TTHEHE AALCOHOLLCOHOL TEXTBOOKEXTBOOK T TH 44TH EEDITIONDITION A reference for the beverage, fuel and industrial alcohol industries Edited by KA Jacques, TP Lyons and DR Kelsall Foreword iii The Alcohol Textbook 4th Edition A reference for the beverage, fuel and industrial alcohol industries K.A. Jacques, PhD T.P. Lyons, PhD D.R. Kelsall iv T.P. Lyons Nottingham University Press Manor Farm, Main Street, Thrumpton Nottingham, NG11 0AX, United Kingdom NOTTINGHAM Published by Nottingham University Press (2nd Edition) 1995 Third edition published 1999 Fourth edition published 2003 © Alltech Inc 2003 All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988. Applications for the copyright holder’s written permission to reproduce any part of this publication should be addressed to the publishers. ISBN 1-897676-13-1 Page layout and design by Nottingham University Press, Nottingham Printed and bound by Bath Press, Bath, England Foreword v Contents Foreword ix T. Pearse Lyons Presient, Alltech Inc., Nicholasville, Kentucky, USA Ethanol industry today 1 Ethanol around the world: rapid growth in policies, technology and production 1 T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA Raw material handling and processing 2 Grain dry milling and cooking procedures: extracting sugars in preparation for fermentation 9 Dave R. Kelsall and T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA 3 Enzymatic conversion of starch to fermentable sugars 23 Ronan F. -

Whiskey Compendium
WHISKEY COMPENDIUM Whiskey is a spirit distilled from fermented grains and aged in oak barrels, which give it most of its colour and flavour. Our whiskey compendium is a humble tribute to this great spirit that is made and enjoyed all around the world. “The water was not fit to drink. To make it palatable, we had to add whiskey. By diligent effort, I learned to like it.” - Winston Churchill - OUR COLLECTION 1 – AMERICAN WHISKEY 2 – TRADITIONAL (RYE) BOURBON 3 – WHEATED BOURBON 4 – SMALL BATCH BOURBON 5 – SINGLE BARREL BOURBON 6 – VINTAGE AND UNIQUE BOURBON 7 – TENNESSEE WHISKEY 8 – RYE WHISKEY 9 – OTHER AMERICAN WHISKEY 10 – SCOTCH WHISKY (WHERE IT ALL BEGAN) 11 – BLENDED WHISKY 12 – SINGLE MALTS OF SCOTLAND (SPEYSIDE) 13 – SINGLE MALTS OF SCOTLAND (HIGHLAND & LOWLAND) 14 – SINGLE MALTS OF SCOTLAND (ISLAY & CAMPBELTOWN) 15 – SINGLE MALTS OF SCOTLAND (THE ISLANDS) 16 – INDEPENDENT BOTTLERS 17 – RARE & PRESTIGIOUS 18 – WHISKEYS OF THE WORLD (IRELAND & CANADA) 19 – WHISKIES OF THE WORLD AMERICAN WHISKEY – 1 – A SHORT HISTORY OF WHISKY OR WHISKEY? AMERICAN WHISKEY The Irish and Americans spell American whiskey is regulated by some whiskey with an “e”. The of the strictest laws of any spirit in Scots, Japanese and Canadians the world. Its heritage began when early spell whisky without. American settlers preserved their extra rye crops by distilling them. Although different to the barley they were used to in Europe, rye still made damn good whiskey. It was in the 18th Centu r y, EASY DISTILLATION when a quarter of a million Scottish and Irish immigrants arrived, that Distillation is a way to separate alcohol whiskey became serious business and from water, by boiling fermented grains the tax collectors were, of course, (called mash) and condensing its vapour. -
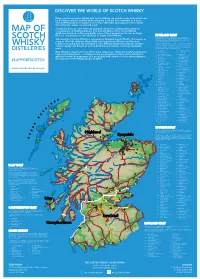
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages
The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages © 2016 Robert A. Freitas Jr. All Rights Reserved. Abstract. Specialized nanofactories will be able to manufacture specific products or classes of products very efficiently and inexpensively. This paper is the first serious scaling study of a nanofactory designed for the manufacture of a specific food product, in this case high-value-per- liter alcoholic beverages. The analysis indicates that a 6-kg desktop appliance called the Fine Spirits Synthesizer, aka. the “Whiskey Machine,” consuming 300 W of power for all atomically precise mechanosynthesis operations, along with a commercially available 59-kg 900 W cryogenic refrigerator, could produce one 750 ml bottle per hour of any fine spirit beverage for which the molecular recipe is precisely known at a manufacturing cost of about $0.36 per bottle, assuming no reduction in the current $0.07/kWh cost for industrial electricity. The appliance’s carbon footprint is a minuscule 0.3 gm CO2 emitted per bottle, more than 1000 times smaller than the 460 gm CO2 per bottle carbon footprint of conventional distillery operations today. The same desktop appliance can intake a tiny physical sample of any fine spirit beverage and produce a complete molecular recipe for that product in ~17 minutes of run time, consuming <25 W of power, at negligible additional cost. Cite as: Robert A. Freitas Jr., “The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages,” IMM Report No. 47, May 2016; http://www.imm.org/Reports/rep047.pdf. 2 Table of Contents 1. -

How to Malt Barley (Or Wheat) for Beer (Or Whisky/Whiskey) I
How to Malt Barley (or Wheat) for Beer (or Whisky/Whiskey) I. Start with Good Quality Barley (or Wheat) A. When purchasing grain for malting, first ask about what types of chemicals might have been used on the grain and then about the grain's history: when it was harvested, how it was stored, etc. B. Inspect the Grain 1. Bits of chaff – and even the occasional dead bug – are quite normal and are of little concern. This sort of debris can easily be removed later when the grain is washed. It will usually float to the surface of the water where it can be skimmed off in a matter of minutes. 2. Grain that has live bugs should generally be avoided, as such bugs will spread to other grain stores and feast on all available grain until it is ready for use. Some of those bugs will also contribute a strong off-smell and off-taste, as well. However, buggy grain can occasionally be had for free, and there are a few types of bugs that do minimal damage to the grain if they are not allowed much time to do their worst. But definitely proceed cautiously with live bugs. 3. The presence of foreign seeds mixed in with the grain may or may not be problematic. A few kernels of corn, a bean or two, a few oats, etc., should not be cause for concern, but if there are unknown seeds mixed in with the grain, ask what they are and act accordingly. 4. Examine the color of the grain and avoid any that has a grayish hue, as this would indicate that the grain was mildewed, either in the field or in storage. -

WHISKEY AMERICAN WHISKEY Angel's Envy Port Barrel Finished
WHISK(E)YS BOURBON WHISKEY AMERICAN WHISKEY Angel's Envy Port Barrel Finished ............................................................ $12.00 High West Campfire Whiskey ................................................................... $10.00 Basil Hayden's ............................................................................................ $12.00 Jack Daniel's ............................................................................................... $8.00 Belle Meade Sour Mash Whiskey ............................................................. $10.00 Gentleman Jack ........................................................................................ $11.00 Belle Meade Madeira Cask Bourbon ........................................................ $15.00 George Dickel No.12 ................................................................................... $9.00 Blackened Whiskey .................................................................................... $10.00 Mitcher's American Whiskey .................................................................... $12.00 Buffalo Trace ............................................................................................... $8.00 Mitcher's Sour Mash Whiskey .................................................................. $12.00 Bulleit Bourbon ............................................................................................ $8.00 CANADIAN WHISKY Bulleit Bourbon 10 year old ...................................................................... $13.00 -

Undestanding Malting Barley Quality Aaron Macleod, Director, Center for Craft Food and Beverage Hartwick College, Oneonta, NY, 13820 [email protected]
Undestanding Malting Barley Quality Aaron MacLeod, Director, Center for Craft Food and Beverage Hartwick College, Oneonta, NY, 13820 [email protected] Malt is the key ingredient in beer that provides the starch and enzymes necessary to produce the fermentable sugars which yeast then turn into alcohol. Malt also provides the color and flavor compounds which contribute to the final character of beer. Malting is the biological process that turns barley into malt. It is a three stage process including soaking (or steeping) the grain in water to bring the kernels to 45% moisture; germination under cool, humid conditions and drying (or kilning) to dry and stabilize the final malt. Barley must meet strict quality criteria to be acceptable for malt production. Maintaining tight controls on these quality factors in the grain is necessary to ensure good processing efficiency and final product quality in the malthouse and brewery. Care must be taken when growing malting barley to ensure that it meets the necessary specifications. A premium is paid to growers for high quality malting barley to compensate for the extra effort. High quality malting barley should have the following characteristics: Pure lot of an acceptable variety Germination of 95% or higher Protein content raging between 9.5% to 12.5% (dry basis) Moisture content below 13.5% Plump and uniform kernels Free of disease and low DON content Less than 5% of peeled, broken, or damaged kernels Clean and free of insects, admixtures, ergot or foreign material Varietal purity Malting barley varieties are bred specifically for characteristics that promote good malting and brewing performance, such as high enzymatic activity, as well as good agronomic performance and disease resistance. -

Questions & Answers
uk g. or y. k is h -w h c t o c s . w w Questions & Answers w The World of Scotch Whisky ORKNEY Kirkwall S E Thurso John D o'Groats I LEWIS R Stornoway Wick B E Lochinver H R Brora Ullapool E Bonar Bridge NORTH T UIST Tain U Invergordon Speyside Torridon O Dingwall Lossiemouth Elgin Portree SOUTH Buckie Banff Forres Fraserburgh UIST Nairn Macduff Keith Inverness Rothes Kyle of Lochalsh Craigellachie SKYE ly eau B ss e Grantown- Huntly N Dufftown h on-Spey c o Fort Augustus L y e Tomintoul Oldmeldrum p S Aviemore Inverurie Mallaig Aberdeen Dee Ballater Fort William Banchory Ben Nevis Loch Ericht 1343 m Stonehaven Tobermory Ballachulish Loch Pitlochry Rannoch Aberfeldy MULL Tay Montrose Blairgowrie Oban Loch Tay Arbroath Loch Awe Carnoustie Perth Dundee Inveraray Crieff Auchterarder Callander Loch Dunblane St Andrews Lochgilphead Lomond Stirling Kinross Glenrothes Helensburgh JURA Falkirk Port Askaig Greenock Dumbarton Tarbert EDINBURGH North ISLAY Berwick GLASGOW Port Ellen East Kilbride Brodick Berwick- Kilmarnock upon-Tweed Campbeltown ARRAN Troon Tweed Biggar Prestwick Melrose Coldstream Ayr Hawick Moffat Dumfries Stranraer Castle Douglas Wigtown Contents Introduction _____________________________________________ 2 - 3 The World's Leading Drink__________________________________ 4 - 7 The History of Scotch Whisky ______________________________ 8 - 10 Making Scotch Whisky ___________________________________ 11 - 19 The Importance of Blending ______________________________ 20 - 22 Scotch Whisky and the World _____________________________