Wetting Properties of Liquid Lithium on Lithium Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Name of the substance Lithium oxide (Li2O) Identification number 235-019-5 (EC number) Synonyms Lithium oxide (Li2O) * Dilithium oxide * LITHIUM OXIDE Document number 1LW Materion Code 1LW Issue date 30-September-2013 Version number 04 Revision date 11-January-2018 Supersedes date 14-July-2015 1.2. Relevant identified uses of the substance or mixture and uses advised against Identified uses Not available. Uses advised against None known. 1.3. Details of the supplier of the safety data sheet Supplier Company name Materion Advanced Chemicals Inc. Address 407 N. 13th Street 1316 W. St. Paul Avenue Milwaukee, WI 53233 United States Division Milwaukee Telephone 414.212.0257 e-mail [email protected] Contact person Noreen Atkinson 1.4. Emergency telephone number SECTION 2: Hazards identification 2.1. Classification of the substance or mixture The substance has been assessed and/or tested for its physical, health and environmental hazards and the following classification applies. Classification according to Regulation (EC) No 1272/2008 as amended Health hazards Skin corrosion/irritation Category 1B H314 - Causes severe skin burns and eye damage. Serious eye damage/eye irritation Category 1 Hazard summary Causes serious eye damage. 2.2. Label elements Label according to Regulation (EC) No. 1272/2008 as amended Contains: Lithium oxide Hazard pictograms None. Signal word None. Hazard statements H314 Causes severe skin burns and eye damage. Precautionary statements Prevention P280 Wear eye protection/face protection. Response P301 + P330 + P331 IF SWALLOWED: rinse mouth. -

Explosion of Lithium-Thionyl-Chloride Battery Due to Presence of Lithium Nitride
Downloaded from orbit.dtu.dk on: Sep 25, 2021 Explosion of lithium-thionyl-chloride battery due to presence of lithium nitride Hennesø, E.; Hedlund, Frank Huess Published in: Journal of Failure Analysis and Prevention Link to article, DOI: 10.1007/s11668-015-0004-y Publication date: 2015 Document Version Early version, also known as pre-print Link back to DTU Orbit Citation (APA): Hennesø, E., & Hedlund, F. H. (2015). Explosion of lithium-thionyl-chloride battery due to presence of lithium nitride. Journal of Failure Analysis and Prevention, 15(5), 600-603. https://doi.org/10.1007/s11668-015-0004-y General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. This article appeared in Journal of Failure Analysis and Prevention, ISSN 1547-7029 http://dx.doi.org/10.1007/s11668-015-0004-y Explosion of lithium-thionyl- chloride battery due to presence of lithium nitride Document no. -

Reactions of Lithium Nitride with Some Unsaturated Organic Compounds. Perry S
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1963 Reactions of Lithium Nitride With Some Unsaturated Organic Compounds. Perry S. Mason Jr Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Mason, Perry S. Jr, "Reactions of Lithium Nitride With Some Unsaturated Organic Compounds." (1963). LSU Historical Dissertations and Theses. 898. https://digitalcommons.lsu.edu/gradschool_disstheses/898 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 64—5058 microfilmed exactly as received MASON, Jr., Perry S., 1938- REACTIONS OF LITHIUM NITRIDE WITH SOME UNSATURATED ORGANIC COMPOUNDS. Louisiana State University, Ph.D., 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. REACTIONS OF LITHIUM NITRIDE WITH SOME UNSATURATED ORGANIC COMPOUNDS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requireiaents for the degree of Doctor of Philosophy in The Department of Chemistry by Perry S. Mason, Jr. B. S., Harding College, 1959 August, 1963 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. -

1 Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction Nikifar Lazouski,1 Zachary J Schiffer,1 Kindle Wi
© 2019 This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ doi: 10.1016/j.joule.2019.02.003 Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction Nikifar Lazouski,1 Zachary J Schiffer,1 Kindle Williams,1 and Karthish Manthiram1* 1Department of Chemical Engineering; Massachusetts Institute of Technology; Cambridge, MA 02139, USA *Corresponding Author: [email protected] 1 © 2019 This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ doi: 10.1016/j.joule.2019.02.003 Summary Ammonia is a large-scale commodity chemical that is crucial for producing nitrogen- containing fertilizers. Electrochemical methods have been proposed as renewable and distributed alternatives to the incumbent Haber-Bosch process, which utilizes fossils for ammonia production. Herein, we report a mechanistic study of lithium-mediated electrochemical nitrogen reduction to ammonia in a non-aqueous system. The rate laws of the main reactions in the system were determined. At high current densities, nitrogen transport limitations begin to affect the nitrogen reduction process. Based on these observations, we developed a coupled kinetic-transport model of the process, which we used to optimize operating conditions for ammonia production. The highest Faradaic efficiency observed was 18.5 ± 2.9%, while the highest production rate obtained was (7.9 ± 1.6) × 10-9 mol cm-2 s-1. Our understanding of the reaction network and the influence of transport provides foundational knowledge for future improvements in continuous lithium- mediated ammonia synthesis. -

Lithium Nitride
TECHNICAL DATA SHEET Date of Issue: 2016/12/12 Lithium Nitride CAS-No. 26134-62-3 EC-No. 247-475-2 Molecular Formula Li3N Product Number 401121 SPECIFICATION Lithium Nitride: min. 94% METHOD OF ANALYSIS Assay by determination of nitrogen by the method of Kjeldahl. A detailed laboratory instruction is available on request. PHYSICAL PROPERTIES Appearance fine powder Color red brown Melting point/ range ca. 840 - 845 °C Density ca. 1.38 g/cm3 at 20 °C Water solubility (Not applicable) Molecular weight 34.82 g/mol Additional Physical Theoretical lithium weight: 59.8 % Properties The information presented herein is believed to be accurate and reliable, but is presented without guarantee or responsibility on the part of Albemarle Corporation and its subsidiaries and affiliates. It is the responsibility of the user to comply with all applicable laws and regulations and to provide for a safe workplace. The user should consider any health or safety hazards or information contained herein only as a guide, and should take those precautions which are necessary or prudent to instruct employees and to develop work practice procedures in order to promote a safe work environment. Further, nothing contained herein shall be taken as an inducement or recommendation to manufacture or use any of the herein materials or processes in violation of existing or future patent. Technical data sheets may change frequently. You can download the latest version from our website www.albemarle-lithium.com. Please contact us at www.albemarle-lithium.com/contact with questions. Lithium Nitride Page 2 / 3 Product Number: 401121 Date of Issue: 2016/12/12 HANDLING & STORAGE Handling Lithium Nitride should be handled under inert gas atmosphere. -

(12) United States Patent (10) Patent No.: US 8,871,843 B2 Lee (45) Date of Patent: Oct
USOO887 1843B2 (12) United States Patent (10) Patent No.: US 8,871,843 B2 Lee (45) Date of Patent: Oct. 28, 2014 (54) HALOGEN-FREE FLAME RETARDANT 5,456,984 A 10/1995 Bishop et al. MATERAL 5,484,830 A 1/1996 Staendeke 5,648.436 A 7/1997 Janowitz et al. 5,925,700 A 7/1999 Imahashi (75) Inventor: Jean L. Lee, San Jose, CA (US) 5,955, 184 A 9, 1999 Honda et al. 5,994,429 A 11/1999 Honda et al. (73) Assignee: Apple Inc., Cupertino, CA (US) 6,140,411 A 10/2000 Schwanborn et al. 6,355,767 B1 * 3/2002 Takagi .......................... 528,196 (*) Notice: Subject to any disclaimer, the term of this 6,440,567 B1 8, 2002 Choate et al. 6,495,244 B1 12/2002 Andresakis et al. patent is extended or adjusted under 35 6,518,336 B1 2/2003 Yabuhara et al. U.S.C. 154(b) by 190 days. 6,642,288 B1 1 1/2003 Hulskotte 6,755,995 B1 6/2004 Hasegawa et al. (21) Appl. No.: 12/638,489 6,767,941 B2 7/2004 Van Der Speket al. 6,809,130 B2 10/2004 Chiou et al. 6,894, 101 B2 5, 2005 Paul et al. (22) Filed: Dec. 15, 2009 6,916,539 B2 7/2005 Cooray et al. 6,998,536 B2 2/2006 Barusseau et al. (65) Prior Publication Data 7,053,145 B1 5/2006 Tasaka et al. US 2011/O144244A1 Jun. 16, 2011 7,115,678 B2 10/2006 Ihara et al. -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: CHEMICAL PRODUCT and COMPANY IDENTIFICATION Product Name: Lithium nitride 99.5% Li -60 Mesh Product Code: L04310 Supplier: Pfaltz & Bauer, Inc. 172 E. Aurora Street Waterbury, CT 06708 USA Phone: 203-574-0075 FAX: 203-574-3181 Emergency Phone: INFOTRAC, US: 1-800-535-5053 INFOTRAC, INTERNATIONAL: +1-352-323-3500 SECTION 2: HAZARDS IDENTIFICATION Statement of Hazard: Corrosive, Dangerous when wet, Irritant, Reacts violently with water, Respiratory irritant Acute Health Hazard: Irritant to eyes, skin, mucous membranes and respiratory system. May be harmful by ingestion, skin absorption and inhalation. Chronic Health Hazard: Not Available HMIS Rating: H: 3 F: 3 P: 2 NFPA Rating: H: 3 F: 0 R: 2 To the best of our knowledge, the toxicological properties of this chemical have not been thoroughly investigated. Use appropriate procedures and precautions to prevent or minimize exposure. GHS Classification in accordance with 29 CFR 1910 (OSHA HCS): Page 1 of 7 Acute toxicity, dermal (Category 4), H312 Acute toxicity, inhalation (Category 4), H332 Acute toxicity, oral (Category 4), H302 Serious eye damage/eye irritation (Category 2A), H319 Skin corrosion/irritation (Category 1A), H314 Specific target organ toxicity, single exposure; Respiratory tract irritation (Category 3), H335 Substances and mixtures which, in contact with water, emit flammable gases (Category 1), H260 Pictogram: Signal Word: Danger Hazard Statement(s): H260 In contact with water releases flammable gases which may ignite spontaneously. H302 Harmful if swallowed. H312 Harmful in contact with skin. H314 Causes severe skin burns and eye damage. H319 Causes serious eye irritation. H332 Harmful if inhaled. -

Low Temperature and Pressure Synthesis of Lithium–Nitride
Materials Transactions, Vol. 54, No. 12 (2013) pp. 2233 to 2237 ©2013 The Japan Institute of Metals and Materials Low Temperature and Pressure Synthesis of LithiumNitride Compound with H2O Addition on Lithium Target for BNCT Shintaro Ishiyama1,+, Yuji Baba1, Ryo Fujii2, Masaru Nakamura2 and Yoshio Imahori2 1Quantum Beam Science Directorate, Japan Atomic Energy Agency, Naka-gun, Ibaraki 319-1195, Japan 2Cancer Intelligence Care Systems, Inc., Tokyo 135-0063, Japan Low temperature synthesis of lithiumnitride compound was conducted on the lithium target for BNCT by N2/H2O mixing gas squirt in the ultra high vacuum chamber, and the following results were derived. (1) Lithiumnitride compound was synthesized on the lithium target ¹8 under 101.3 Pa N2 gas squirt at room temperature and in the ultra high vacuum chamber under the pressure of 1 © 10 Pa. (2) Remarkable contamination by O and C was observed on the lithiumnitride compound synthesized under the squirt pressure of 13.380 Pa/1.334.7 Pa N2/ H2O mixing gas. (3) No contamination and synthesis of LiN compound was observed under the squirt pressure of 0.0130.027 Pa/00.005 Pa N2/H2O mixing gas. (4) Contamination by O and C was enhanced with excessive addition of H2O at the pressure of over 1.33 Pa. [doi:10.2320/matertrans.M2013242] (Received June 26, 2013; Accepted September 25, 2013; Published November 9, 2013) Keywords: boron neutron capture therapy, neutron source, lithium target, lithium nitride, nitrogen gas, contamination, H2O addition 1. Introduction Implemented deployment of accelerator-driven neutron source for Boron Neutron Capture Therapy (BNCT) is scheduled in 2013 in National Cancer Center, Japan. -

Uncertain Material Experiment Size in Industry
'10.5045 JULY 9. 1966 NATURE 125 The problem of nucleation is considered by V. N. Fili and certainly no information, about the reliability of povich by discussing a very simple model of a mixture some of the experimental results, in a field in which of two particles. As he says, " ... success depends on the reproducibility is not to be taken for granted. Figs. extent to which the model reflects the real system. The 45, 56 and 81 provide typical examples of justifiable development of a detailed theory of crystallization of doubts concerning the data. The theoret.ical calculations complex glasses and melts directly depends on the state seem to be similarly fraught with difficulties, and it is no of the theory of liquid and glass structure, which is still surprise to find a note added in proof which, concerning far from perfection". the displacement energy Ta, says "It now appears that The editor of this volume, E. A. Porai-Koshits, has 60 e Vis a better value than 25 e V, and if this is accepted, made important contributions to the study of glasses by the number of displaced atoms given in this book should X-ray diffraction and his laboratory has for some years be reduced by the ratio 25: 60". now concentrated on the use of small-angle X-ray diffrac The author has achieved a very creditable performance tion, light scattering and electron microscopy to study the with his cast of eccentrics and has provided a valuable, development of immiscibility in simple glasses and the up-to-date story of his field. -

Gemological ABSTRACTS 2002
Gemological ABSTRACTS 2002 EDITOR COLORED STONES AND A. A. Levinson ORGANIC MATERIALS University of Calgary Characterization of beryl (aquamarine variety) by Mössbauer Calgary, Alberta, Canada spectroscopy. R. R. Viana, G. M. da Costa, E. De Grave, H. Jordt-Evangelista, and W. B. Stern, Physics and REVIEW BOARD Chemistry of Minerals, Vol. 29, No. 1, 2002, pp. 78–86. Five aquamarine samples were analyzed by Mössbauer spec- Anne M. Blumer troscopy to find a correlation between their dark blue to green- Bloomington, Illinois ish blue colors and the locations of iron atoms in the beryl Jo Ellen Cole structure. An asymmetric Fe2+ doublet was observed in the Vista, California spectra of all samples at room temperature. The asymmetry is D. Darmour related to a relaxation process involving Fe2+ ions and water GIA Gem Trade Laboratory, Carlsbad molecules in structural channels. At higher temperatures, the Vladislav Dombrovskiy spectra indicated at least two Fe2+ components. At very low GIA Gem Trade Laboratory, Carlsbad temperatures, the spectra of a deep blue specimen showed that 2+ 2+ R. A. Howie Fe was in structural channels. Fe also occupied octahedral Royal Holloway, University of London and tetrahedral sites, whereas Fe3+ was only located in the octa- hedral site. The authors conclude that the color of green-to-blue Alethea Inns 3+ GIA Gem Trade Laboratory, Carlsbad beryls is determined by the relative proportions of Fe in the octahedral sites and of Fe2+ in the channels. Thus, deep blue Taijin Lu beryls have little Fe3+, whereas greener beryls have more octahe- GIA Research, Carlsbad dral Fe3+ or less channel Fe2+. -

The Ionic Conductivity in Lithium-Boron Oxide Materials and Its Relation to Structural, Electronic and Defect Properties: Insights from Theory
Journal of Physics: Condensed Matter TOPICAL REVIEW Related content - Topical Review The ionic conductivity in lithium-boron oxide Paul Heitjans and Sylvio Indris - Double perovskites with ferromagnetism materials and its relation to structural, electronic above room temperature and defect properties: insights from theory D Serrate, J M De Teresa and M R Ibarra - How chemistry controls electron localization in 3d1 perovskites: a Wannier- To cite this article: Mazharul M Islam et al 2012 J. Phys.: Condens. Matter 24 203201 function study E Pavarini, A Yamasaki, J Nuss et al. Recent citations View the article online for updates and enhancements. - Some device implications of voltage controlled magnetic anisotropy in Co/Gd 2 O 3 thin films through REDOX chemistry Guanhua Hao et al - Lithium Diffusion Mechanisms in -LiMO2 (M = Al, Ga): A Combined Experimental and Theoretical Study Mazharul M. Islam et al - First-principles study of structural, electronic, energetic and optical properties of substitutional Cu defect in Li 2 B 4 O 7 scintillator C. Santos et al This content was downloaded from IP address 134.129.67.237 on 13/06/2018 at 22:54 IOP PUBLISHING JOURNAL OF PHYSICS: CONDENSED MATTER J. Phys.: Condens. Matter 24 (2012) 203201 (29pp) doi:10.1088/0953-8984/24/20/203201 TOPICAL REVIEW The ionic conductivity in lithium-boron oxide materials and its relation to structural, electronic and defect properties: insights from theory Mazharul M Islam1,2, Thomas Bredow1,2 and Paul Heitjans2,3 1 Mulliken Center for Theoretical Chemistry, Universitat¨ -
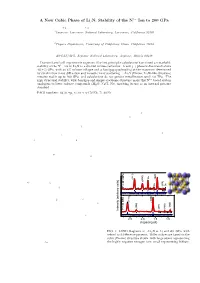
A New Cubic Phase of Li3n: Stability of the N3− Ion to 200
3¡ A New Cubic Phase of Li3N: Stability of the N Ion to 200 GPa A. Lazicki1;2, B. Maddox1;2, W. J. Evans, C. -S. Yoo and A. K. McMahan 1Lawrence Livermore National Laboratory, Livermore, California 94550 W. E. Pickett and R. T. Scalettar 2Physics Department, University of California, Davis, California 95616 M. Y. Hu and P. Chow HPCAT/APS, Argonne National Laboratory, Argonne, Illinois 60439 Diamond anvil cell experiments augmented by ¯rst principles calculations have found a remarkable 3¡ stability of the N ion in Li3N to a six-fold volume reduction. A new (γ) phase is discovered above 40(§5) GPa, with an 8% volume collapse and a bandgap quadrupling at the transition determined by synchrotron x-ray di®raction and inelastic x-ray scattering. γ-Li3N (Fm3m, Li3Bi-like structure) remains stable up to 200 GPa, and calculations do not predict metallization until »8 TPa. The high structural stability, wide bandgap and simple electronic structure make this N3¡ based system analogous to lower valency compounds (MgO, NaCl, Ne), meriting its use as an internal pressure standard. PACS numbers: 62.50.+p, 61.10.-i, 64.70.Kb, 71.20.Nr Nitrogen can form compounds with elements from al- particular interest. most every column in the periodic table, with chemi- In this paper, we present the ¯rst concrete experimen- cal bonding ranging from covalent to ionic to metallic. tal evidence that ¯-Li3N indeed transforms to a cubic Lithium nitride is the only known thermodynamically structure (γ-Li3N) in the pressure range of 36-45 GPa. stable alkali metal nitride and is one of the most ionic This transformation represents an increase in structural of all known nitrides.