Recognition and Management of Pesticide Poisonings: Sixth Edition: 2013: Chapter 19 Misc PSSA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow -

Hydrogen Sulfide Metabolite, Sodium Thiosulfate
International Journal of Molecular Sciences Review Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms Max Y. Zhang 1,2, George J. Dugbartey 1,2,3, Smriti Juriasingani 1,3 and Alp Sener 1,2,3,4,* 1 Matthew Mailing Center for Translational Transplant Studies, London Health Sciences Center, Western University, London, ON N6A 5A5, Canada; [email protected] (M.Y.Z.); [email protected] (G.J.D.); [email protected] (S.J.) 2 London Health Sciences Center, Multi-Organ Transplant Program, Western University, London, ON N6A 5A5, Canada 3 London Health Sciences Center, Department of Surgery, Division of Urology, Western University, London, ON N6A 5A5, Canada 4 Department of Microbiology & Immunology, Schulich School of Medicine & Dentistry, University of Western Ontario, London, ON N6A 3K7, Canada * Correspondence: [email protected]; Tel.: +1(519) 6633352 Abstract: Thiosulfate in the form of sodium thiosulfate (STS) is a major oxidation product of hydrogen sulfide (H2S), an endogenous signaling molecule and the third member of the gasotransmitter family. STS is currently used in the clinical treatment of acute cyanide poisoning, cisplatin toxicities in cancer therapy, and calciphylaxis in dialysis patients. Burgeoning evidence show that STS has antioxidant and anti-inflammatory properties, making it a potential therapeutic candidate molecule that can target multiple molecular pathways in various diseases and drug-induced toxicities. This review Citation: Zhang, M.Y.; Dugbartey, discusses the biochemical and molecular pathways in the generation of STS from H2S, its clinical G.J.; Juriasingani, S.; Sener, A. usefulness, and potential clinical applications, as well as the molecular mechanisms underlying these Hydrogen Sulfide Metabolite, clinical applications and a future perspective in kidney transplantation. -

Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children
Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children • 1 million cases of exposure to toxins in children younger than 6 years reported in the U.S. In 1993 • estimated that another 1 million exposures to toxins not reported • 1% have moderate or major life-threatening symptoms • 60-100 deaths annually in the U.S Poisoning in Children Less Than 5 Years Old • accounts for 85-90% of pediatric poisoning • is generally accidental • secondary to exploratory behavior and lack of supervision • tend to involve single agent ingestions Poisoning in Children Over 5 Years Old • accounts for 10-15% of pediatric poisoning • is generally intentional • secondary to suicide attempts or gestures, or to intoxications and inadvertent overdose • tend to involve multiple agent ingestions General Concepts for Pediatric Poisoning • Prevention • Initial Stabilization • Diagnosis • Specific Antidotes Management of the Poisoned Child • Treat the Patient, Not the Poison --patient-specific treatment is safer, less expensive, and more effective Management of the Poisoned Child • Stabilization --Airway --Breathing --Circulation --Disability (neurologic) Management of the Poisoned Child • Respiratory Failure --airway obstruction from secretions, refluxed gastric contents, airway muscle relaxation --respiratory muscle rigidity --loss of respiratory drive --pulmonary edema Management of the Poisoned Child • Cardiovascular Collapse --arteriolar dilation --venous dilation --myocardial depression --dysrhythmias Management of the Poisoned Child • Neurologic -

Martin Steinhoff Dirk Roosterman, Tobias Goerge, Stefan W
Dirk Roosterman, Tobias Goerge, Stefan W. Schneider, Nigel W. Bunnett and Martin Steinhoff Physiol Rev 86:1309-1379, 2006. doi:10.1152/physrev.00026.2005 You might find this additional information useful... This article cites 963 articles, 265 of which you can access free at: http://physrev.physiology.org/cgi/content/full/86/4/1309#BIBL Medline items on this article's topics can be found at http://highwire.stanford.edu/lists/artbytopic.dtl on the following topics: Biochemistry .. Transient Receptor Potential Channel Biochemistry .. Endopeptidases Biochemistry .. Proteolytic Enzymes Oncology .. Inflammation Medicine .. Neurogenic Inflammation Physiology .. Nerves Updated information and services including high-resolution figures, can be found at: http://physrev.physiology.org/cgi/content/full/86/4/1309 Downloaded from Additional material and information about Physiological Reviews can be found at: http://www.the-aps.org/publications/prv This information is current as of February 8, 2008 . physrev.physiology.org on February 8, 2008 Physiological Reviews provides state of the art coverage of timely issues in the physiological and biomedical sciences. It is published quarterly in January, April, July, and October by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2005 by the American Physiological Society. ISSN: 0031-9333, ESSN: 1522-1210. Visit our website at http://www.the-aps.org/. Physiol Rev 86: 1309–1379, 2006; doi:10.1152/physrev.00026.2005. Neuronal Control of Skin Function: The Skin as a Neuroimmunoendocrine Organ DIRK ROOSTERMAN, TOBIAS GOERGE, STEFAN W. SCHNEIDER, NIGEL W. BUNNETT, AND MARTIN STEINHOFF Department of Dermatology, IZKF Mu¨nster, and Boltzmann Institute for Cell and Immunobiology of the Skin, University of Mu¨nster, Mu¨nster, Germany; and Departments of Surgery and Physiology, University of California, San Francisco, California I. -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

A Review of Hyperacusis and Future Directions: Part I. Definitions and Manifestations
AJA Review Article A Review of Hyperacusis and Future Directions: Part I. Definitions and Manifestations Richard S. Tyler,a Martin Pienkowski,b Eveling Rojas Roncancio,a Hyung Jin Jun,a Tom Brozoski,c Nicolas Dauman,d Claudia Barros Coelho,a Gerhard Andersson,e,f Andrew J. Keiner,a Anthony T. Cacace,g Nora Martin,a and Brian C. J. Mooreh Purpose: Hyperacusis can be extremely debilitating, and at Results: Hyperacusis encompasses a wide range of present, there is no cure. We provide an overview of the reactions to sound, which can be grouped into the field, and possible related areas, in the hope of facilitating categories of excessive loudness, annoyance, fear, and future research. pain. Many different causes have been proposed, and it will Method: We review and reference literature on be important to appreciate and quantify different subgroups. hyperacusis and related areas. We have divided the Reasonable approaches to assessing the different forms of review into 2 articles. In Part I, we discuss definitions, hyperacusis are emerging, including psychoacoustical epidemiology, different etiologies and subgroups, and measures, questionnaires, and brain imaging. how hyperacusis affects people. In Part II, we review Conclusions: Hyperacusis can make life difficult for many, measurements, models, mechanisms, and treatments, forcing sufferers to dramatically alter their work and social and we finish with some suggestions for further habits. We believe this is an opportune time to explore research. approaches to better understand and treat hyperacusis. yperacusis can be devastating for those who suf- refereed publications, books, and conference proceedings. fer from it. This review is intended to clarify what We highlighted what we believe are key issues that are im- H is known at present about hyperacusis and its portant to move forward, sometimes even drawing from underlying mechanisms to focus research and to promote areas not normally associated with hyperacusis. -
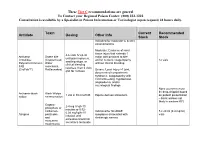
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Interview and History Taking Strategies CHAPTER 2 Physical Examination Strategies CHAPTER 3 Documentation Strategies
Part 1 Strategies for Eff ective Health Assessment CHAPTER 1 Interview and History Taking Strategies CHAPTER 2 Physical Examination Strategies CHAPTER 3 Documentation Strategies 1 © Jones & Bartlett Learning, LLC, an Ascend Learning Company. NOT FOR SALE OR DISTRIBUTION 9781284131390_CH01_Pass01.indd 1 24/09/16 12:24 PM Chapter 1 Interview and History Taking Strategies “In taking histories follow each line of thought, ask no leading questions. Never suggest. Give the patient’s own words in the complaint.” Sir William Osler (1849–1919) (Bean & Bean, 1968) Functions of the Interview and Health History Interviewing and taking health histories serve fi ve major functions: 1. Establishing the initial bond between provider and patient (Figure 1-1) 2. Laying the foundation for subsequent clinical decision making 3. Providing a legal record of the subjective and objective data (Box 1-1) elicited during the clinical interview, which drive clinical judgments 4. Fulfi lling a critical component of the documentation required for third-party payer reimbursement for clinical services 5. Serving as an essential element in the peer review process for evaluation of clinical practice, such as application of evidence-based practice and identifi cation of desired patient outcomes As the primary goal of this text is to help the reader to develop expertise in advanced health assessment, this chapter will focus primarily on functions one and two. Legal and reimbursement requirements mandate meticulous, comprehensive, and complete documentation of all the components of care, including patient teaching and counsel- ing provided at each provider–patient encounter. These include not only the traditional face-to-face encounters, but also other means of care, such as interaction via e-mail and telephone. -

Functional Sensory Symptoms
Handbook of Clinical Neurology, Vol. 139 (3rd series) Functional Neurologic Disorders M. Hallett, J. Stone, and A. Carson, Editors http://dx.doi.org/10.1016/B978-0-12-801772-2.00024-2 © 2016 Elsevier B.V. All rights reserved Chapter 24 Functional sensory symptoms J. STONE1* AND M. VERMEULEN2 1Department of Clinical Neurosciences, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK 2Department of Neurology, Academic Medical Center, Amsterdam, The Netherlands Abstract Functional (psychogenic) sensory symptoms are those in which the patient genuinely experiences alter- ation or absence of normal sensation in the absence of neurologic disease. The hallmark of functional sen- sory symptoms is the presence of internal inconsistency revealing a pattern of symptoms governed by abnormally focused attention. In this chapter we review the history of this area, different clinical presentations, diagnosis (including sensitivity of diagnostic tests), treatment, experimental studies, and prognosis. Altered sensation has been a feature of “hysteria” since descriptions of witchcraft in the middle ages. In the 19th century hysteric sensory stigmata were considered a hallmark of the condition. Despite this long history, relatively little attention has been paid to the topic of functional sensory disturbance, compared to functional limb weakness or functional movement disorders, with which it commonly coexists. There are recognizable clinical patterns, such as hemisensory disturbance and sensory disturbance fin- ishing at the groin or shoulder, but in keeping with the literature on reliability of sensory signs in neurology in general, the evidence suggests that physical signs designed to make a positive diagnosis of functional sensory disorder may not be that reliable. -

Prognosis of a Case with Paresthesia Associated with Prolonged Touching of an Endodontic Paste to the Inferior Alveolar Nerve
J Clin Exp Dent. 2011;3(Suppl1):e377-81. Inferior alveolar nerve paresthesia. Journal section: Oral Surgery doi:10.4317/jced.3.e377 Publication Types: Case Report Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve M. Cemil Buyukkurt 1, Hakan Arslan 2, H. Sinan Topcuoglu 2, M. Melih Omezli 3 1 PhD, DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Sifa University, İzmir, Turkey 2 DDS. Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, Turkey 3 DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ataturk University, Erzurum, Turkey Correspondence: Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, 25240, Turkey E-mail address: [email protected] Received: 10/02/2011 Accepted: 08/05/2011 Buyukkurt MC, Arslan H, Topcuoglu HS, Omezli MM. Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve. J Clin Exp Dent. 2011;3(Suppl1):e377- 81. http://www.medicinaoral.com/odo/volumenes/v3iSuppl1/jcedv3iSu- ppl1p377.pdf Article Number: 50506 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Abstract Paresthesia is described as an abnormal sensation, such as burning, pricking, tickling, tingling, formication or num- bness. Several conditions can cause paresthesia. This article presents a case of paresthesia caused by the extrusion of endodontic paste (Endomethasone®) into the mandibular canal. The clinical manifestations comprised the numb- ness on the right side of the mandible and right lower lip, appearing after endodontic treatment. -

Dialyzability of Medications During Intermittent Hemodialysis
DialyzeIHD: Dialyzability of Medications During Intermittent Hemodialysis % Dialyzed % Dialyzed % Dialyzed % Dialyzed IHD Dosing; Administration IHD Dosing; Administration IHD Dosing; Administration IHD Dosing; Administration Timing Drug (Type of Drug (Type of Drug (Type of Drug (Type of Timing Around HD Session Timing Around HD Session Around HD Session Dialyzer) Timing Around HD Session Dialyzer) Dialyzer) Dialyzer) 0.25-0.5mg PO Q8H PRN, Insulin Aspart, Pentamidine 0 4mg/kg IV Q24-36H, Not recommended for use, Clonazepam N/A Reduce to 25-50% of normal dose Acarbose N/A Administer anytime during HD Insulin Detemir, Isethionate (N/A) Administer anytime during HD Administer anytime during HD N/A and titrate, Administer anytime Insulin Glargine, 0.1-0.4mg PO Q8-12H, during HD Normal dose and titrate based on 100-150mg PO Q12-24H, <5 Insulin Lispro Acebutolol N/A Clonidine Administer anytime during HD; No target free or corrected total Administer post-HD (Low Flux) Phenytoin Dose post-HD if hypotensive 75-300mg PO Q24H, (Low Flux) phenytoin level, Normal dose based on indication, 0 Administer anytime during HD Acetaminophen N/A 75mg PO Q24H, Irbesartan Administer anytime during HD; Dose Administer anytime during HD Clopidogrel N/A (N/A) Administer anytime during HD post-HD if hypotensive No 15-45mg PO Q24H, Pioglitazone 2.5-5mg/kg IV/PO Q24H, (N/A) Administer anytime during HD 40-60 250-500mg PO Q6H or 1-2g IV Q4- 100mg IV weekly to monthly, Acyclovir Administer post-HD over 60 Cloxacillin N/A Iron Dextran N/A (N/A) 6H, Administer anytime