Evaluating the Impact of Predation by Fish on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congolli (Pseudaphritis Urvillii) and Australian Salmon (Arripis Truttaceus and A
Inland Waters and Catchment Ecology Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 SARDI Aquatics Sciences PO Box 120 Henley Beach SA 5022 September 2015 Giatas and Ye (2015) Diet of three fish species in the Coorong Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 September 2015 II Giatas and Ye (2015) Diet of three fish species in the Coorong This publication may be cited as: Giatas, G.C. and Ye, Q. (2015). Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2015/000479-1. SARDI Research Report Series No. 858. 81pp. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.pir.sa.gov.au/research DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Research Chief, Aquatic Sciences. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Table of Fishes of Sydney Harbour 2019
Table of Fishes of Sydney Harbour 2019 Family Family/Com Species Species Common Notes mon Name Name Acanthuridae Surgeonfishe Acanthurus Eyestripe close s dussumieri Surgeonfish to southern li mit Acanthuridae Acanthurus Orangebloch close to olivaceus Surgeonfish southern limit Acanthuridae Acanthurus Convict close to triostegus Surgeonfish southern limit Acanthuridae Acanthurus Yellowmask xanthopterus Surgeonfish Acanthuridae Paracanthurus Blue Tang not included hepatus in species count Acanthuridae Prionurus Spotted Sawtail maculatus Acanthuridae Prionurus Australian Sawtail microlepidotus Ambassidae Glassfishes Ambassis Port Jackson jacksoniensis glassfish Ambassidae Ambassis marianus Estuary Glassfish Anguillidae Freshwater Anguilla australis Shortfin Eel Eels Anguillidae Anguilla reinhardtii Longfinned Eel Antennariidae Anglerfishes Antennarius Freckled Anglerfish southern limit coccineus Antennariidae Antennarius Giant Anglerfish close to commerson southen limit Antennariidae Antennarius Shaggy Anglerfish southern limit hispidus Antennariidae Antennarius pictus Painted Anglerfish Antennariidae Antennarius striatus Striate Anglerfish Table of Fishes of Sydney Harbour 2019 Antennariidae Histrio histrio Sargassum close to Anglerfish southen limit Antennariidae Porophryne Red-fingered erythrodactylus Anglerfish Aploactinidae Velvetfishes Aploactisoma Southern Velvetfish milesii Aploactinidae Cocotropus Patchwork microps Velvetfish Aploactinidae Paraploactis Bearded Velvetfish trachyderma Aplodactylidae Seacarps Aplodactylus Rock Cale -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

The Corner Inlet Fishery
The Corner Inlet Fishery Information to inform assessment of the Victorian Corner Inlet Fishery under the Environment Protection and Biodiversity Conservation Act 1999 © The State of Victoria Department of Economic Development, Jobs, Transport and Resources This work is licensed under a Creative Commons Attribution 3.0 Australia licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo and the Department of Environment and Primary Industries logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Accessibility If you would like to receive this publication in an alternative format, please contact the Customer Service Centre at 136 186 or [email protected] or the National Relay Service on 133 677 or www.relayservice.com.au . Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. Contents Introduction ............................................................................................................................................................... 2 Description -

Biology, Stock Status and Management Summaries for Selected Fish Species in South-Western Australia
Fisheries Research Report No. 242, 2013 Biology, stock status and management summaries for selected fish species in south-western Australia Claire B. Smallwood, S. Alex Hesp and Lynnath E. Beckley Fisheries Research Division Western Australian Fisheries and Marine Research Laboratories PO Box 20 NORTH BEACH, Western Australia 6920 Correct citation: Smallwood, C. B.; Hesp, S. A.; and Beckley, L. E. 2013. Biology, stock status and management summaries for selected fish species in south-western Australia. Fisheries Research Report No. 242. Department of Fisheries, Western Australia. 180pp. Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Fisheries Western Australia. While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Department of Fisheries Western Australia does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. Fish illustrations Illustrations © R. Swainston / www.anima.net.au We dedicate this guide to the memory of our friend and colleague, Ben Chuwen Department of Fisheries 3rd floor SGIO Atrium 168 – 170 St Georges Terrace PERTH WA 6000 Telephone: (08) 9482 7333 Facsimile: (08) 9482 7389 Website: www.fish.wa.gov.au ABN: 55 689 794 771 Published by Department of Fisheries, Perth, Western Australia. Fisheries Research Report No. 242, March 2013. ISSN: 1035 - 4549 ISBN: 978-1-921845-56-7 ii Fisheries Research Report No.242, 2013 Contents ACKNOWLEDGEMENTS ............................................................................................... -

National Strategy for the Survival of Released Line-Caught Fish: a Review of Research and Fishery Information
NATIONAL STRATEGY FOR THE SURVIVAL OF RELEASED LINE-CAUGHT FISH: A REVIEW OF RESEARCH AND FISHERY INFORMATION FRDC Project 2001/101 McLeay, L.J, Jones, G.K. and Ward, T.M. November 2002 South Australian Research and Development Institute (SARDI) PO Box 120, Henley Beach, South Australia 5022 ISBN 0730852830 NATIONAL STRATEGY FOR THE SURVIVAL OF RELEASED LINE-CAUGHT FISH: A REVIEW OF RESEARCH AND FISHERY INFORMATION McLeay, L.J, Jones, G.K. and Ward, T.M. November 2002 Published by South Australian Research and Development Institute (Aquatic Sciences) © Fisheries Research and Development Corporation and SARDI. This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. DISCLAIMER The authors do not warrant that the information in this report is free from errors or omissions. The authors do not accept any form of liability, be it contractual, tortious or otherwise, for the contents of this report or for any consequences arising from its use or any reliance placed upon it. The information, opinions and advice contained in this report may not relate to, or be relevant to, a reader’s particular circumstances. Opinions expressed by the authors are the individual opinions of those persons and are not necessarily those of the publisher or research provider. ISBN No. 0730852830 TABLE -

Print 1950-04-17 IPFC Sec II.Tif (106 Pages)
SOME POPULATIONS OF THE AUSTRALIAN "SALMON", Arripis trutta by the Late W. S. air bridge* ABSTRACT Australian salmon occur in large shoals, usually A preliminary account is glvell of the lnvesti atioll close inshore. They have the habit of coming close of thc Australun Salmon (Arripis truth) p~?cipaf ly as into the beach while in these shoals, especially in an examination of the distribution (m a1 hfephases) certain localities, and it is then that the fishermen of different stocks of the species along the Australian catch them. coast, and the taxononnc status of thesc stocks. ,Mor- bolo iml and othcr evidence 1s presented to support an This is usually regarded as an onshore species. EyptLis that the stocks constitute distinct ncs with Shoals are not commonly sighted beyond the conti- separate breeding grounds. nental shelf; but there are reports, apparently reliable, The Australian salmon is a percomorph, and of salmon shoals being sighted, and specimens caught, not in any way related to the Salmonidae. The well out in the Tasman Sea, popular name was presumably given to it by the Figure 1 shows the general world distribution early settlers in this country, on the strength of some of the species. In Australia it extends up the east superficial resemblances to the true salmons and and west coasts to about latitude 30°S, though trouts. occasionally it is taken very much further north. The present paper is only a progress report. The There is a large* alld apparently quite untapped, results are not yet fully tested and definite, but it population of in New It Occurs was thought that delegates might be interested as also in the waters around Norfolk Island and Lord much in the lines along which the work is progress- HOwe Island. -

Fisheries Resources in Otago Harbour and on the Adjacent Coast
Fisheries resources in Otago Harbour and on the adjacent coast Prepared for Port Otago Limited December 2008 R O Boyd Boyd Fisheries Consultants Ltd 1 Baker Grove Wanaka 9305 NEW ZEALAND Table of Contents Table of Contents i List of Tables and Figures ii Executive Summary iii Fisheries resources in Otago Harbour and on the adjacent coast 1 (Ver 1 – Preliminary) 1 1. Introduction 1 2. Methods 2 2.1. The Fisheries Literature 2 2.2. Fisheries catch and effort data 2 2.3. Consultation with the Fisheries Sector (this section to be revised following further consultation) 2 3. The Fisheries Environments of Otago Harbour and Coastal Otago 3 3.1. Otago Harbour 3 3.2. Coastal Otago 3 4. Fish and Shellfish Fauna of Otago Harbour and Coastal Otago 5 4.1. Otago Harbour 5 4.2. Coastal Otago 6 4.3. Regional and National Significance 7 5. Areas of Importance for Spawning, Egg Laying or Juveniles 8 6. Fisheries Uses of Otago Harbour and Coastal Otago 9 6.1. Recreational Fisheries 9 6.1.1. Otago Harbour 9 6.1.2. Coastal Otago 10 6.2. Commercial Fisheries 10 6.2.1. History and Background to the Commercial Fishery 10 6.2.2. Commercial Fisheries Catch and Effort Data 11 6.2.3. Overview of the Present Otago Commercial Fishery 12 6.2.4. Otago’s Inshore Fisheries 13 Trawl fishery 13 Set net fishery 14 Cod potting 14 Line fishing 14 Paua and kina diving 14 Queen scallops 15 Rock lobster 15 Cockles 15 6.3. Customary Fisheries (this section to be revised following further consultation) 16 7. -

Links Between Seagrass Habitats, Piscivorous Fishes and Their Fish Prey
MELBOURNE Links between seagrass habitats, piscivorous fishes and their fish prey Authors Dr Jeremy S. Hindell Dr Michael J. Keough Dr Gregory P. Jenkins Project No. 1999/215 F I S H E R I E S R E S E A R C H & DEVELOPMENT CORPORATION Fisheries Research and Development Corporation Title Links between seagrass habitats, piscivorous fishes and their fish prey Authors Hindell, J.S.' Keough, M.J.' Jenkins, G.P.2 Address 'Department of Zoology University of Melbourne PARKVILLE, 3010 2 Marine and Freshwater Resources Institute Weeroona Parade QUEENSCLIFF, 3225 September, 2000 ISBN: The information contained in this document is solely for the use of the client for the purpose for which it has been prepared and no representation is to be made or is to be implied as being made to any third party. Pj FRDC 1999/215 Links between predatory fishes and seagrass Table of Contents TABLE OF CONTENTS Non Technical Summary .................................................................................................... 5 Background.......................................................................................................................... 8 Need .................................................................................................................................... 12 Objectives........................................................................................................................... 13 Methods............................................................................................................................. -

Draft Fisheries Plan North-East Finfish Fishery
DRAFT FISHERIES PLAN NORTH-EAST FINFISH FISHERY December 2007 Snapper (SNA 1) Grey mullet (GMU 1) Kahawai (KAH 1) Bluenose (BNS 1) Red gurnard (GUR 1) Kingfish (KIN 1) Pilchard (PIL 1) School shark (SCH 1) Tarakihi (TAR 1) Rig (SPO 1) Flatfish (FLA 1) John dory (JDO 1) Trevally (TRE 1) Häpuku/bass (HPB 1) CONTENTS CONTENTS ..........................................................................................................................................................2 INTRODUCTION ................................................................................................................................................3 EXECUTIVE SUMMARY ............................................................................................................................................3 PURPOSE OF THE NORTH -EAST FINFISH PLAN .........................................................................................................3 SCOPE OF THE NORTH -EAST FINFISH PLAN .............................................................................................................4 FISHERIES IN THE PLAN ...........................................................................................................................................6 SECTION 1: THE CURRENT SITUATION.....................................................................................................9 THE HEALTH OF THE AQUATIC ENVIRONMENT IS PROTECTED ................................................................................9 North-East Coast Marine Environment -
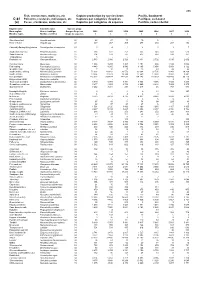
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
495 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southwest C-81 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoccidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 28 27 13 10 5 ... ... River eels nei Anguilla spp 22 337 267 209 277 210 207 152 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 0 4 1 2 1 1 7 Southern lemon sole Pelotretis flavilatus 31 238 322 251 335 348 608 513 Sand flounders Rhombosolea spp 31 204 193 187 437 514 530 351 Tonguefishes Cynoglossidae 31 3 - - - - - - Flatfishes nei Pleuronectiformes 31 2 580 2 986 2 729 3 431 2 702 3 015 2 602 Common mora Mora moro 32 1 308 1 234 1 403 1 154 986 1 180 1 088 Red codling Pseudophycis bachus 32 4 443 8 265 9 540 8 165 5 854 5 854 6 122 Grenadier cod Tripterophycis gilchristi 32 7 10 13 13 43 29 26 Southern blue whiting Micromesistius australis 32 72 203 43 812 26 576 30 304 32 735 23 943 29 268 Southern hake Merluccius australis 32 13 834 22 623 19 344 12 560 12 858 13 892 8 881 Blue grenadier Macruronus novaezelandiae 32 215 302 209 414 147 032 134 145 119 329 103 489 96 119 Ridge scaled rattail Macrourus carinatus 32 - - - - - 9 14 Thorntooth grenadier Lepidorhynchus denticulatus 32 5 349 5 304 6 341 3 855 4 056 3 725 3 264 Grenadiers, rattails nei Macrouridae 32 3 877 4 253 3 732 2 660 2 848 7 939 8 970 Gadiformes nei Gadiformes 32 3 252 3 281 298 1 217 46 767 886 Broadgilled hagfish Eptatretus cirrhatus 33 2 - 0 0 11 508 347 Sea catfishes nei Ariidae 33 4 6 4 4 4 ..