Habitats and Areas of Particular Significance for Coastal Finfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Combined!Effects!In!Europe's!Seas!
OCP/EEA/NSS/18/002.ETC/ICM!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!ETC/ICM!Technical!Report!4/2019! Multiple!pressures!and!their! combined!effects!in!Europe’s!seas! Prepared!by!/!compiled!by:!! Samuli!Korpinen,!Katja!Klančnik! Authors:!! Samuli Korpinen & Katja Klančnik (editors), Monika Peterlin, Marco Nurmi, Leena Laamanen, Gašper Zupančič, Ciarán Murray, Therese Harvey, Jesper H Andersen, Argyro Zenetos, Ulf Stein, Leonardo Tunesi, Katrina Abhold, GerJan Piet, Emilie Kallenbach, Sabrina Agnesi, Bas Bolman, David Vaughan, Johnny Reker & Eva Royo Gelabert ETC/ICM'Consortium'Partners:'' Helmholtz!Centre!for!Environmental!Research!(UFZ),!Fundación!AZTI,!Czech!Environmental! Information!Agency!(CENIA),!Ioannis!Zacharof&!Associates!Llp!Hydromon!Consulting! Engineers!(CoHI(Hydromon)),!Stichting!Deltares,!Ecologic!Institute,!International!Council!for! the!Exploration!of!the!Sea!(ICES),!Italian!National!Institute!for!Environmental!Protection!and! Research!(ISPRA),!Joint!Nature!Conservation!Committee!Support!Co!(JNCC),!Middle!East! Technical!University!(METU),!Norsk!Institutt!for!Vannforskning!(NIVA),!Finnish!Environment! Institute!(SYKE),!Thematic!Center!for!Water!Research,!Studies!and!Projects!development!! (TC!Vode),!Federal!Environment!Agency!(UBA),!University!Duisburg.Essen!(UDE)! ! ! ! Cover'photo! ©!Tihomir!Makovec,!Marine!biology!station!Piran,!Slovenia! ' Layout! F&U!confirm,!Leipzig! ! Legal'notice' The!contents!of!this!publication!do!not!necessarily!reflect!the!official!opinions!of!the!European!Commission!or!other!institutions! -

Reef Fish Monitoring Te Tapuwae O Rongokako Marine Reserve
Reef Fish Monitoring Te Tapuwae o Rongokako Marine Reserve Technical Support - Marine East Coast Hawke’s Bay Conservancy Debbie Freeman OCTOBER 2005 Published By Department of Conservation East Coast Hawkes Bay Conservancy PO Box 668 Gisborne 4040, New Zeland Cover: Banded wrasse Photo: I. Nilsson Title page: Koheru Photo: M. Blackwell Acknowledgments: Blue maomao Photo: J. Quirk © Copyright October 2005, New Zealand Department of Conservation ISSN 1175-026X ISBN 978-0-478-14143-6 (paperback) ISBN 978-0-478-14193-1 (Web pdf) Techincal Support Series Number: 25 In the interest of forest conservation, DOC Science Publishing supports paperless electronic publishing. When printing, recycled paper is used wherever possible. C ontents Abstract 4 Introduction 5 Methods 6 Results 10 Discussion 20 Fish fauna 20 Protection effects 21 Reserve age and design 21 Experimental design and monitoring methods 22 Illegal fishing 23 Environmental factors 24 Acknowledgements 24 References 25 Abstract Reef fish monitoring was undertaken within and surrounding Te Tapuwae o Rongokako Marine Reserve, on the North Island’s East Coast, between 2000 and 2004. The objective of the monitoring was to describe the reef fish communities and to establish whether populations within the marine reserve were demonstrating any changes in abundance or size that could be attributable to the removal of fishing pressure. The underwater visual census method was used to survey the four lo- cations (marine reserve and three non-reserve locations). It was found that all four locations were characterised by moderate densities of spot- ties, scarlet wrasse and reef-associated planktivores such as blue maomao, sweep and butterfly perch. -

Multiple!Pressures!And!Their! Combined!Effects!In!Europe's!Seas!
OCP/EEA/NSS/18/002.ETC/ICM!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!ETC/ICM!Technical!Report!4/2019! Multiple!pressures!and!their! combined!effects!in!Europe’s!seas! Prepared!by!/!compiled!by:!! Samuli!Korpinen,!Katja!Klančnik! Authors:!! Monika Peterlin, Marco Nurmi, Leena Laamanen, Gašper Zupančič, Andreja Popit, Ciarán Murray, Therese Harvey, Jesper H Andersen, Argyro Zenetos, Ulf Stein, Leonardo Tunesi, Katrina Abhold, GerJan Piet, Emilie Kallenbach, Sabrina Agnesi, Bas Bolman, David Vaughan, Johnny Reker & Eva Royo Gelabert ETC/ICM'Consortium'Partners:'' Helmholtz!Centre!for!Environmental!Research!(UFZ),!Fundación!AZTI,!Czech!Environmental! Information!Agency!(CENIA),!Ioannis!Zacharof&!Associates!Llp!Hydromon!Consulting! Engineers!(CoHI(Hydromon)),!Stichting!Deltares,!Ecologic!Institute,!International!Council!for! the!Exploration!of!the!Sea!(ICES),!Italian!National!Institute!for!Environmental!Protection!and! Research!(ISPRA),!Joint!Nature!Conservation!Committee!Support!Co!(JNCC),!Middle!East! Technical!University!(METU),!Norsk!Institutt!for!Vannforskning!(NIVA),!Finnish!Environment! Institute!(SYKE),!Thematic!Center!for!Water!Research,!Studies!and!Projects!development!! (TC!Vode),!Federal!Environment!Agency!(UBA),!University!Duisburg.Essen!(UDE)! ! ! ! Cover'photo! ©!Tihomir!Makovec,!Marine!biology!station!Piran,!Slovenia! ' Layout! F&U!confirm,!Leipzig! ! Legal'notice' The!contents!of!this!publication!do!not!necessarily!reflect!the!official!opinions!of!the!European!Commission!or!other!institutions! of!the!European!Union.!Neither!the!European!Environment!Agency,!the!European!Topic!Centre!on!Inland,!Coastal!and!Marine! -

(2013) Population Genetic Structures of Three Congeneric Species of Co
MURDOCH RESEARCH REPOSITORY This is the author’s final version of the work, as accepted for publication following peer review but without the publisher’s layout or pagination. The definitive version is available at http://dx.doi.org/10.1007/s10641-012-0105-3 Moore, G.I. and Chaplin, J.A. (2013) Population genetic structures of three congeneric species of coastal pelagic fishes (Arripis: Arripidae) with extensive larval, post-settlement and adult movements. Environmental Biology of Fishes, 96 (9). pp. 1087-1099. http://researchrepository.murdoch.edu.au/17252/ Copyright: © 2013 Springer Science+Business Media Dordrech. It is posted here for your personal use. No further distribution is permitted. 1 Population genetic structures of three congeneric species of coastal pelagic fishes (Arripis: 2 Arripidae) with extensive larval, post-settlement and adult movements. 3 4 G.I. Moore1,2 and J.A. Chaplin1 5 6 1 Centre for Fish and Fisheries Research, School of Biological Sciences and Biotechnology, Murdoch 7 University, South St, Murdoch, Western Australia, Australia, 6150. 8 2 Fish Section, Department of Aquatic Zoology, Western Australian Museum, 49 Kew St., Welshpool, 9 Western Australia, Australia, 6106. 10 11 Author to whom correspondence should be addressed: 12 G.I. Moore 13 e-mail: [email protected] 14 phone: +61 08 9212 3744 15 1 16 Abstract 17 18 The population genetic structures of three congeneric coastal pelagic marine fishes (Arripis trutta, A. 19 truttaceus and A. georgianus) were investigated to determine whether these structures were consistent 20 with the apparently high gene flow life histories of these species. -

The Osmoregulatory Metabolism Op the Starry Flounder, Platichthys Stellatus
THE OSMOREGULATORY METABOLISM OP THE STARRY FLOUNDER, PLATICHTHYS STELLATUS by CLEVELAND PENDLETON HICKMAN, JR. B.A., DePauw University, 1950 M.S., University of New Hampshire, 1953 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of Zoology We accept this thesis as conforming to the required standard. THE UNIVERSITY OF BRITISH COLUMBIA June, 1958 Faculty of Graduate Studies PROGRAMME OF THE FINAL ORAL EXAMINATION FOR THE DEGREE OF DOCTOR OF PHILOSOPHY of CLEVELAND PENDLETON HICKMAN JR. B.A. DePauw University, 1950 M.S. University of New Hampshire, 1953 IN ROOM 187A, BIOLOGICAL SCIENCES BUILDING MONDAY, JUNE 30, 1958 at 10:30 a.m. COMMITTEE IN CHARGE DEAN F. H. SOWARD, Chairman H. ADASKIN W. S. HOAR W. A. CLEMENS W. N. HOLMES I. McT. COWAN C. C. LINDSEY P. A. DEHNEL H. McLENNAN R. F. SCAGEL External Examiner: F. E. J. FRY University of Toronto THE OSMOREGULATORY METABOLISM OF THE STARRY FLOUNDER, PLATICHTYS STELLATUS ABSTRACT Energy demands for osmotic regulation and the possible osmoregulatory role of the thyroid gland were investigated in the euryhaline starry flounder, Platichthys stellatus. Using a melt• ing-point technique, it was established that flounder could regulate body fluid concentration independent of widely divergent environ• mental salinities. Small flounder experienced more rapid disturb• ances of body fluid concentration than large flounder after abrupt salinity alterations. The standard metabolic rate of flounder adapted to fresh water was consistently and significantly less than that of marine flounder. In supernormal salinities standard metabolic rate was significantly greater than in normal sea water. -

To Next File: Sfc260a.Pdf
Appendix 1 FISH SPECIES IDENTIFIED FROM THE COASTAL EAST CAPE REGION Surveys of the East Cape Region (Bay of Plenty and East Coast) were conducted during 1992, 1993, and 1999 (see Tables 1, 2, and 4 for locality data), with additional records from the National Fish Collection. FAMILY, SPECIES, AND AUTHORITY COMMON NAME STATIONS Lamnidae Isurus oxyrinchus Rafinesque mako shark (P) E20o Squalidae Squalus mitsukurii Jordon & Snyder piked spurdog (D) W Dasyatidae Dasyatis brevicaudata (Hutton) shorttailed stingray E19o Myliobatidae eMyliobatis tenuicaudatus (Hector) eagle ray E03° Anguillidae Anguilla australis Richardson shortfin eel (F) W eAnguilla dieffenbachii Gray longfin eel (F) W Muraenidae Gymnothorax prasinus (Richardson) yellow moray E05o, E08° Ophichthyidae Scolecenchelys australis (MacLeay) shortfinned worm eel E01, E10, E11, E28, E29, E33, E48 Congridae Conger verreauxi Kaup southern conger eel E03, E07, E09, E12°, E13, E16, E18, E22o, E23o, E25o, E31, E33 Conger wilsoni (Bloch & Schneider) northern conger eel W; E05, E09, E12, E27 Conger sp. conger E01°, E31o Engraulidae Engraulis australis (White) anchovy (P) E Clupeidae Sardinops neopilchardus (Steindachner) pilchard (P) E Gonorynchidae Gonorynchus forsteri Ogilby sandfish (D) E Retropinnidae eRetropinna retropinna (Richardson) smelt (F) W; E Galaxiidae Galaxias maculatus (Jenyns) inanga (F) W; E Bythitidae eBidenichthys beeblebroxi Paulin grey brotula E01, E03, E08, E09, E10, E12, E18, E19, E21, E23, E27, E28, E29, E31, E33, E34 eBrosmodorsalis persicinus Paulin & Roberts pink brotula E02, E07, E10°, E18, E22, E29, E31, E33o eDermatopsis macrodon Ogilby fleshfish E08, E31 Moridae Austrophycis marginata (Günther) dwarf cod (D) E Continued next page > e = NZ endemic species. D = deepwater species (> 50 m depth); E = estuarine species; F = freshwater species; P = pelagic species; U = species of uncertain identity. -

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

A Checklist of Fishes of the Aldermen Islands, North-Eastern New Zealand, with Additions to the Fishes of Red Mercury Island
13 A CHECKLIST OF FISHES OF THE ALDERMEN ISLANDS, NORTH-EASTERN NEW ZEALAND, WITH ADDITIONS TO THE FISHES OF RED MERCURY ISLAND by Roger V. Grace* SUMMARY Sixty-five species of marine fishes are listed for the Aldermen Islands, and additions made to an earlier list for Red Mercury Island (Grace, 1972), 35 km to the north. Warm water affinities of the faunas are briefly discussed. INTRODUCTION During recent years, and particularly the last four years, over 30 species of fishes have been added to the New Zealand fish fauna through observation by divers, mainly at the Poor Knights Islands (Russell, 1971; Stephenson, 1970, 1971; Doak, 1972; Whitley, 1968). A high proportion of the fishes of northern New Zealand have strong sub-tropical affinities (Moreland, 1958), and there is considerable evidence (Doak, 1972) to suggest that many of the recently discovered species are new arrivals from tropical and subtropical areas. These fishes probably arrive as eggs or larvae, carried by favourable ocean currents, and find suitable habitats for their development at the Poor Knights Islands, where the warm currents that transported the young fish or eggs maintain a water temperature higher than that on the adjacent coast, or islands to the south. Unless these fishes are able to establish breeding populations in New Zealand waters, they are likely to be merely transient. If they become established, they may begin to spread and colonise other off-shore islands and the coast. In order to monitor any spreading of new arrivals, or die-off due to inability to breed, it is desirable to compile a series of fish lists, as complete as possible, for the off-shore islands of the north-east coast of New Zealand. -

Congolli (Pseudaphritis Urvillii) and Australian Salmon (Arripis Truttaceus and A
Inland Waters and Catchment Ecology Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 SARDI Aquatics Sciences PO Box 120 Henley Beach SA 5022 September 2015 Giatas and Ye (2015) Diet of three fish species in the Coorong Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 September 2015 II Giatas and Ye (2015) Diet of three fish species in the Coorong This publication may be cited as: Giatas, G.C. and Ye, Q. (2015). Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2015/000479-1. SARDI Research Report Series No. 858. 81pp. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.pir.sa.gov.au/research DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Research Chief, Aquatic Sciences. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. -
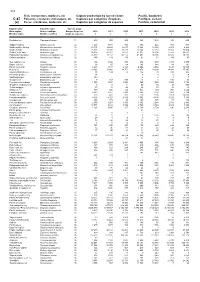
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Catch and Effort Data
Chapter 11: The Inland Waters River Fishery Information System CHAPTER 11: INLAND WATERS RIVER FISHERY INFORMATION SYSTEM 11.1 Background The Inland Waters River fishery is a multi-species, multi-gear fishery encompassing the South Australian sector of the River Murray and its backwaters (Map 11.1). Historically, the fishery was based on harvesting Murray cod (Maccullochella peelii peelii), golden perch (Macquaria ambigua), and bony bream (Nematalosa erebi). The fishery was restructured in July 2003 to a non-native dominate fishery and the commercial fishing for significant native species is now prohibited. The fishery is now based predominantly on the taking European carp (Cyprinus carpio) and redfin perch (Perca fluviatilis). There are a total of 6 licence holders who operate within the fishery. Prior to the restructure 30 license holders fished the river, each with a designated reach. Up to December 2007 SARDI production tables hold in excess of 270,000 daily records. Data is held from 1984/85 to current and is continually being updated. Refer to Figure 11.3 for an entity relationship diagram of the inland waters system that encompasses the River Fishery. South Australian Aquatic Sciences: Information Systems and Database Support Program SARDI Aquatic Sciences Information Systems Quality Assurance and Data Integrity Report (June 2009) 81 Chapter 11: The Inland Waters River Fishery Information System Map 11.1: The inland waters river fishery – River Murray area designations. South Australian Aquatic Sciences: Information Systems and Database Support Program SARDI Aquatic Sciences Information Systems Quality Assurance and Data Integrity Report (June 2009) 82 Chapter 11: The Inland Waters River Fishery Information System 11.2 Research Logbook Information Each licence holder is required to submit a daily fishing return by the 15th day of each month detailing their fishing activities for the previous month (Figure 11.1). -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences.