World Database for Pediatric and Congenital Heart Surgery Appendix A: Surgical Procedure Terms and Definitions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
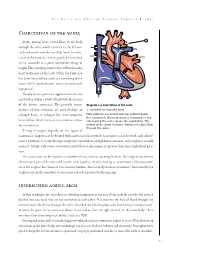
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Surgery for Acquired Heart Disease
View metadata, citation and similar papers at core.ac.uk brought to you byCORE provided by Elsevier - Publisher Connector SURGERY FOR ACQUIRED HEART DISEASE EARLY RESULTS WITH PARTIAL LEFT VENTRICULECTOMY Patrick M. McCarthy, MD a Objective: We sought to determine the role of partial left ventriculectomy in Randall C. Starling, MD b patients with dilated cardiomyopathy. Methods: Since May 1996 we have James Wong, MBBS, PhD b performed partial left ventriculectomy in 53 patients, primarily (94%) in Gregory M. Scalia, MBBS b heart transplant candidates. The mean age of the patients was 53 years Tiffany Buda, RN a Rita L. Vargo, MSN, RN a (range 17 to 72 years); 60% were in class IV and 40% in class III. Marlene Goormastic, MPH c Preoperatively, 51 patients were thought to have idiopathic dilated cardio- James D. Thomas, MD b myopathy, one familial cardiomyopathy, and one valvular cardiomyopathy. Nicholas G. Smedira, MD a As our experience accrued we increased the extent of left ventriculectomy James B. Young, MD b and more complex mitral valve repairs. For two patients mitral valve replacement was performed. For 51 patients the anterior and posterior mitral valve leaflets were approximated (Alfieri repair); 47 patients also had ring posterior annuloplasty. In 27 patients (5!%) one or both papillary muscles were divided, additional left ventricular wall was resected, and the papillary muscle heads were reimplanted. Results: Echocardiography showed a significant decrease in left ventricular dimensions after resection (8.3 cm to 5.8 cm), reduction in mitral regurgitation (2.8+ to 0), and increase in forward ejection fraction (15.7% to 32.7%). -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

Reduction Ventriculoplasty for Dilated Cardiomyopathy : the Batista Procedure Shahram Salemy Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1999 Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure Shahram Salemy Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Salemy, Shahram, "Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure" (1999). Yale Medicine Thesis Digital Library. 3123. http://elischolar.library.yale.edu/ymtdl/3123 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. SlDDCITOM VENTRICULOPIASTy FOR DILATED CARDIOMYOPATHY THE BATISTA PROCEDURE W«M * (e,yx»> ShaLramSalemy YALE DNIVERSriY YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. Signature of Author Date REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE Shahram Salemy B.S., George Tellides M.D., Ph.D., and John A. Elefteriades M.D. February 5, 1999 r 113 f'Uh (e(e.cl 0 REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE. -

Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G
ORIGINAL ARTICLES: CONGENITAL HEART SURGERY CONGENITAL HEART SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal. CONGENITAL HEART Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G. Co-Vu, MD,* Salil Ginde, MD,* Peter J. Bartz, MD, Peter C. Frommelt, MD, James S. Tweddell, MD, and Michael G. Earing, MD Department of Pediatrics, Division of Pediatric Cardiology, and Department of Internal Medicine, Division of Cardiovascular Medicine, and Department of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, Wisconsin Background. After the arterial switch operation (ASO) score increased at an average rate of 0.08 per year over for transposition of the great arteries (TGA), the native time after ASO. Freedom from neoaortic root dilation at pulmonary root and valve function in the systemic posi- 1, 5, 10, and 15 years after ASO was 84%, 67%, 47%, and tion, and the long-term risk for neoaortic root dilation 32%, respectively. Risk factors for root dilation include -pre ,(0.003 ؍ and valve regurgitation is currently undefined. The aim history of double-outlet right ventricle (p and length of ,(0.01 ؍ of this study was to determine the prevalence and pro- vious pulmonary artery banding (p Neoaortic valve regurgitation of at .(0.04 ؍ gression of neoaortic root dilation and neoaortic valve follow-up (p regurgitation in patients with TGA repaired with the least moderate degree was present in 14%. -

TGA Surgical Techniques: Tips & Tricks
TGA Surgical techniques: tips & tricks (Arterial switch operation) Seoul National University Children’s Hospital Woong-Han Kim Surgical History • 1951 Blalock and Hanlon, atrial septectomy • 1954 Mustard et al. arterial switch op • (monkey lung, 7 patients, 19 days old) • 1958 Senning, Atrial switch operation • 1963 Mustard, Mustard operation • 1966 Raskind and Miller, Balloon atrial septostomy • 1969 Rastelli, Rastelli operation • 1975 Jatene, first successulful ASO in patients with TGA and large VSD • 1977 Yacoub et al. two stage repair • 1983 Quaegebeur and Castaneda, primary repair in neonate • 1988 Boston group, rapid two-stage ASO The wide range of spatial relationships between the great arteries in TGA d-TGA Taussig-Bing (DORV) Posterior TGA Complete Transposition of the Great Arteries . Ventriculoarterial discordance . Also known as d-TGA (d = dextroposition of the bulboventricular loop) . Aorta on the right and anterior • Morphogenesis – Failure of the septum to spiral • Straight septum • Parallel arrangement of RVOT and LVOT – Abnormal growth and development of subaortic infundibulum – Absence of subpulmonic infundibulum growth Major coexisting anomalies * About 75% of neonates presenting TGA have no other cardiac anomalies, other than PFO or ASD and 20% have VSD and only 5% have LVOTO. 1 VSD Conoventricular(not necessarily juxtapulmonary) 55~60% Juxtaaortic 5% Juxtaarterial 5% Inlet septal 5% Juxtatricuspid Juxtacrucial : straddling muscular 25~30% 2 LVOTO It occurs 0.7% in TGA + IVS at birth and 20% in TGA+VSD, and may develop after birth in others and so reach an overall prevalence of 30%. Dynamic type - leftward bulging of septum Anatomic type - subvalvar fibrous ridge, fibrous tags, aneurysm, muscular (malalignment) or fibromuscular obstruction, valvar hypoplasia, combined. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -

Prenatal Diagnosis, Associated Findings and Postnatal Outcome Of
J. Perinat. Med. 2019; 47(3): 354–364 Ingo Gottschalka,*, Judith S. Abela, Tina Menzel, Ulrike Herberg, Johannes Breuer, Ulrich Gembruch, Annegret Geipel, Konrad Brockmeier, Christoph Berg and Brigitte Strizek Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center https://doi.org/10.1515/jpm-2018-0316 anomalies, 30 (66.7%) had extracardiac anomalies and 13 Received September 18, 2018; accepted November 26, 2018; (28.9%) had chromosomal or syndromal anomalies. Due previously published online December 20, 2018 to their complex additional anomalies, five (11.1%) of our Abstract 45 fetuses had multiple malformations and were highly suspicious for non-chromosomal genetic syndromes, Objective: To assess the spectrum of associated anoma- although molecular diagnosis could not be provided. Dis- lies, the intrauterine course, postnatal outcome and orders of laterality occurred in 10 (22.2%) fetuses. There management of fetuses with double outlet right ventricle were 17 terminations of pregnancy (37.8%), two (4.4%) (DORV). intrauterine and seven (15.6%) postnatal deaths. Nineteen Methods: All cases of DORV diagnosed prenatally over a of 22 (86.4%) live-born children with an intention to treat period of 8 years were retrospectively collected in a single were alive at last follow-up. The mean follow-up among tertiary referral center. All additional prenatal findings survivors was 32 months (range, 2–72). Of 21 children who were assessed and correlated with the outcome. The accu- had already undergone postnatal surgery, eight (38.1%) racy of prenatal diagnosis was assessed. achieved biventricular repair and 13 (61.9%) received Results: Forty-six cases of DORV were diagnosed pre- univentricular palliation. -

Curriculum Vitae Takahiro Shiota, MD, Phd, FACC, FESC, FASE, FAHA
1 Curriculum Vitae Takahiro Shiota, MD, PhD, FACC, FESC, FASE, FAHA Office Address: Cedars-Sinai Medical Center Heart Institute 127 S. San Vicente Blvd., A3411 Los Angeles, CA 90048 (310) 423-6889 Office Email: [email protected] EDUCATION: 1991 Ph.D. in Cardiology. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1977-1983 M.D. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1972-1976 B.S. in Physics. Faculty of Science, University of Tokyo, Tokyo, Japan LICENSURE AND CERTIFICATION National Board of Echocardiography (#2000-252) California Medical License (#000015) Ohio Medical License (#35. 080318) ECFMG (#0-576-045-9) Japanese Medical License (#274951) PROFESSIONAL EXPERIENCE 1/2009-present Associate Director Division of Noninvasive Cardiology Cedars-Sinai Heart Institute Los Angeles, CA 12/2001-12/2008 Clinical Staff Department of Cardiovascular Medicine Cleveland Clinic, Cleveland, OH 7/1999-11/2001 Advanced Cardiac Department of Cardiovascular Medicine Imaging Fellow Cleveland Clinic, Cleveland, OH 9/1997- 6/1999 Project Staff Department of Cardiovascular Medicine 2 Cleveland Clinic, Cleveland, OH 8/1992- 8/1997 Research Director Cardiac Imaging Laboratory, Clinical Care Center for Congenital Heart Disease, Oregon Health Sciences University, Portland, OR PROFESSIONAL ACTIVITIES: Academic Appointment 7/2009-present Professor of Medicine, Department of Medicine, Cedars-Sinai, Los Angeles, CA 8/2008-present Clinical Professor of Medicine, David Geffen School of Medicine at UCLA 7/2007-12/2008 Professor of Medicine, Cleveland -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

A Focus on Valve-Sparing Ascending Aortic Aneurysm Repair Newyork
ADVANCES IN CARDIOLOGY, INTERVENTIONAL CARDIOLOGY, AND CARDIOVASCULAR SURGERY Affiliated with Columbia University College of Physicians and Surgeons and Weill Cornell Medical College A Focus on Valve-Sparing NOVEMBER/DECEMBER 2014 Ascending Aortic Aneurysm Repair Emile A. Bacha, MD The most frequent location for aneurysms in the Chief, Division of Cardiac, chest occurs in the ascending aorta – and these Thoracic and Vascular Surgery aneurysms are often associated with either aortic NewYork-Presbyterian/Columbia stenosis or aortic insufficiency, especially when the University Medical Center aneurysm involves a bicuspid aortic valve. Director, Congenital and Pediatric Cardiac Surgery “We know that patients who have enlarged NewYork-Presbyterian Hospital aortas or aneurysms of the ascending aorta are at [email protected] great risk for one of two major life-threatening events: an aortic rupture or an aortic dissection,” Allan Schwartz, MD says Leonard N. Girardi, MD, Director of Chief, Division of Cardiology Thoracic Aortic Surgery in the Department of NewYork-Presbyterian/Columbia Cardiothoracic Surgery, NewYork-Presbyterian/ University Medical Center Weill Cornell Medical Center. “Dissection of the Valve-sparing ascending aortic aneurysm repair [email protected] inner lining of the wall of the blood vessel can also lead to rupture or other complications down last 15 years, the Aortic Surgery Program at Weill O. Wayne Isom, MD the line. For example, as the tear extends it may Cornell has been aggressively pursuing the devel- Cardiothoracic Surgeon-in-Chief NewYork-Presbyterian/ affect the vessels that supply the brain or the opment of a procedure that would enable surgeons Weill Cornell Medical Center coronary arteries or cause tremendous damage to to spare the patient’s native valve. -

101St Annual Meeting Share Your Annual Meeting Experience on Twitter: Featuring Aortic Symposium @AATSHQ and Mitral Conclave #AATS2021 a Virtual Learning Experience
TABLE OF CONTENTS AATS Annual Meeting Committees ............................................................................. 2 Accreditation ......................................................................................................................... 5 Scientific Program ...............................................................................................................8 Abstracts ............................................................................................................................108 C. Walton Lillehei Abstracts ..................................................................................341 Case Video Abstracts ..............................................................................................350 PROGRAM BOOK 101st Annual Meeting Share your Annual Meeting experience on Twitter: Featuring Aortic Symposium @AATSHQ and Mitral Conclave #AATS2021 A Virtual Learning Experience April 30-May 2, 2021 This program book went to ePrint on April 29, 2021. Any program changes made after this date are available at aats.org/annualmeeting. President Marc R. Moon *AATS Member ◆AATS New Member 1 101st Annual Meeting AMERICAN ASSOCIATION April 30 – May 2, 2021 | A Virtual Learning Experience FOR THORACIC SURGERY AORTIC SYMPOSIUM AATS – PROMOTING SCHOLARSHIP IN Co-Chairs THORACIC AND CARDIOVASCULAR SURGERY *Joseph S. Coselli *Steven L. Lansman Since 1917, when it was founded as the first organization dedicated to thoracic surgery, the Committee Members American Association for Thoracic Surgery