CYANOTIC CONGENITAL HEART DISEASE by JAMES W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
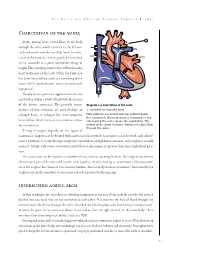
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Reoperation for Tricuspid Regurgitation After Total Correction of Tetralogy of Fallot
Original Reoperation for Tricuspid Regurgitation after Total Article Correction of Tetralogy of Fallot Yoshikazu Hachiro, MD, Nobuyuki Takagi, MD, Tetsuya Koyanagi, MD, and Tomio Abe, MD Background: The aim of this study is to review the outcome of reoperation for severe tricus- pid regurgitation after repair of tetralogy of Fallot . Methods: Between 1972 and 2000, 12 patients underwent reoperation on the tricuspid valve after total correction of tetralogy of Fallot. The mean age at the time of reoperation was 17 years (range, 1 to 39 years). The mean interval between the initial correction and the reoperation was 7.8 years (range, 10 days to 19 years). The functional class was New York Heart Association class II in 2 patients and class III or IV in 10. Six patients underwent tricuspid valve repair, and the others underwent tricuspid valve replacement. Results: Hospital mortality was 16.7% (2/12). Three patients (30%, 3/10) required a second reoperation 1.6, 9.2, and 15.6 years after the most recent reoperation with no deaths. The rea- sons for second reoperation were failure of the tricuspid valve repair in two and a thrombosed valve in one. There were two late deaths. Mean overall event-free actuarial survival at 10 years was 46.3%. Conclusion: Reoperation for severe tricuspid regurgitation after total correction of tetralo- gy of Fallot was associated with a high operative mortality and disappointing long-term results. Tricuspid regurgitation after corrective surgery for tetralogy of Fallot must be diag- nosed promptly and cured, as tolerance is poor because of postoperative right ventricular insufficiency. -
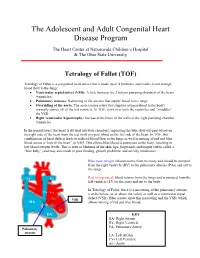
Tetralogy of Fallot (TOF)
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children’s Hospital & The Ohio State University Tetralogy of Fallot (TOF) Tetralogy of Fallot is a congenital heart defect that is made up of 4 problems and results in not enough blood flow to the lungs: • Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles) • Pulmonary stenosis: Narrowing of the arteries that supply blood to the lungs • Overriding of the aorta: The aorta (major artery that supplies oxygen blood to the body) normally comes off of the left ventricle. In TOF, it sits over both the ventricles and “straddles” the VSD. • Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) In the normal heart, the heart is divided into four chambers, separating the blue (low-oxygen) blood on the right side of the heart from the red (with oxygen) blood on the left side of the heart. In TOF, this combination of heart defects leads to reduced blood flow to the lungs as well as mixing of red and blue blood across a “hole in the heart” or VSD. This allows blue blood to pump out to the body, resulting in low blood oxygen levels. This is seen as blueness of the skin, lips, fingernails and tongue (often called a “blue baby”) and may also result in poor feeding, growth problems, and activity intolerance. Blue (low-oxygen) blood returns from the body and should be pumped from the right ventricle (RV) to the pulmonary arteries (PAs) and out to Aorta the lungs. -
Before Reading the Specific Defect
Tetralogy of Fallot What is it? Tetralogy of Fallot refers to a combination of abnormalities with four key features: 1) A ventricular septal defect (a hole between the ventricles) and 2) obstruction of blood flow from the right ventricle to the lungs (pulmonary stenosis) are the most important. Sometimes the pulmonary valve isn’t just narrowed but is completely obstructed (pulmonary atresia). Also, 3) the aorta (major artery from the heart to the body) lies directly over the ventricular septal defect and 4) the right ventricle develops hypertrophy (thickened muscle). Because of the pulmonary stenosis, blood can’t get to the lungs easily, so the blood doesn’t get as much oxygen as it should. Because the aorta overrides the ventricular septal defect, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body. People with unrepaired tetralogy of Fallot are often blue (cyanotic) because of the oxygen-poor blood that’s pumped to the body. What causes it? In most cases, the cause isn’t known although in some patients, genetic factors play a role. It’s a common type of heart defect. It may be seen more commonly in patients with Down syndrome (in association with AV canal defects) or DiGeorge syndrome. Some patients can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a patient with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -
Tetralogy of Fallot
Tetralogy of Fallot What is it? Tetralogy of Fallot has four key features. A ventricular septal defect (VSD; a hole between the ventricles) and obstruction from the right ventricle to the lungs (pulmonary stenosis) are the most important. Also, the aorta (the major artery from the heart to the body) lies directly over the ventricular septal defect, and the right ventricle develops thickened muscle. What causes it? In most children, the cause of tetralogy of Fallot isn’t known. It’s a common type of heart defect. It may be seen more commonly in children with Down syndrome or DiGeorge syndrome. Some children can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a child with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). Obstruction in the pulmonary valve leading from the right ventricle to the lung artery prevents the normal amount of blood from being pumped to the lungs. Sometimes the pulmonary valve is completely obstructed (pulmonary atresia). How does tetralogy of Fallot affect my child? Infants and young children with unrepaired tetralogy of Fallot are often blue (cyanotic). The reason is that some oxygen-poor blood is pumped to the body through the hole in the wall between the right and left ventricle instead of being pumped to the lungs. -

Anomalies of the Aorta and Pulmonary Arteries Complicating Ventricular Septal Defect
Br Heart J: first published as 10.1136/hrt.24.3.279 on 1 May 1962. Downloaded from ANOMALIES OF THE AORTA AND PULMONARY ARTERIES COMPLICATING VENTRICULAR SEPTAL DEFECT BY J. MORGAN, R. PITMAN, J. F. GOODWIN, R. E. STEINER, AND A. HOLLMAN From the Departments of Medicine and Radiodiagnosis, Postgraduate Medical School and Hammersmith Hospital Received August 15, 1961 Ventricular septal defect is not always a simple lesion, but may be associated with other congenital cardiac disorders. The object of this paper is to present anomalies of the great vessels that have been found on angiocardiography or at operation in a series of patients with a ventricular septal defect investigated and treated at Hammersmith Hospital. During the last three years selective angiocardiography has been performed on 165 patients diagnosed as having ventricular septal defect. In 24 of them associated anomalies of the aorta and pulmonary arteries have been found. These patients will be presented in three groups according to the type of anomalies found; Group 1, corrected transposition of the great vessels, Group 2, double outlet right ventricle, Group 3, anomalies of the pulmonary arteries. 1. CORRECTED OF THE GREAT VESSELS GROUP TRANSPOSITION http://heart.bmj.com/ In this anomaly there is anatomical transposition. Viewed from above the aorta is anterior and to the left and the pulmonary trunk posterior and to the right. This is corrected functionally by bulboventricular inversion. Corrected transposition is usually associated with other defects, but the circulation is normal, for venous blood enters the pulmonary circulation and arterial blood the systemic circulation. The systemic ventricle from which the aorta arises has the anatomical features of a right ventricle, and the venous ventricle, from which the pulmonary artery arises, has the anatomical characteristics of a left ventricle. -

Tricuspid Atresia Associated with Common Arterial Trunk and 22Q11 Chromosome Deletion
Archivos de Cardiología de México Volumen Número Octubre-Diciembre Volume 73 Number 4 October-December 2003 Artículo: Tricuspid atresia associated with common arterial trunk and 22q11 chromosome deletion Derechos reservados, Copyright © 2003 Instituto Nacional de Cardiología Ignacio Chávez Otras secciones de Others sections in este sitio: this web site: ☞ Índice de este número ☞ Contents of this number ☞ Más revistas ☞ More journals ☞ Búsqueda ☞ Search edigraphic.com 271 COMUNICACIONES BREVES Tricuspid atresia associated with common arterial trunk and 22q11 chromosome deletion Carlos Alva,* Felipe David,* Martha Hernández,* Rubén Argüero,** José Ortegón,* Arturo Martínez,* Diana López,* Santiago Jiménez,* Agustín Sánchez* Summary Resumen The case of a four-months old male with coexist- ATRESIA TRICUSPÍDEA Y TRONCO ARTERIOSO COMÚN ent tricuspid atresia and common arterial trunk is presented. The diagnois was made by cardiac Se presenta el caso de un paciente masculino catheterization and selective angiocardiography. de cuatro meses de edad con atresia tricuspí- Clinical considerations are discussed and the dea asociada a tronco arterial común. El diag- review of the available literature reveals this pa- nóstico se corroboró con cateterismo cardíaco y tient to be the tenth case reported of this very angiocardiografía selectiva. Se analiza la expre- unusual association of cardiovascular defects, sión clínica de esta excepcional asociación y se and the first with positive deletion of the 22q11 hace una revisión de la literatura. Este es el dé- chromosome. cimo caso publicado con esta inusual asociación de lesiones cardíacas y el primero con deleción del cromosoma 22q11. (Arch Cardiol Mex 2003; 73:271-274). Key words: Tricuspid atresia and common arterial trunk. -

Tricuspid Valve Guideline
Tricuspid Valve What the Nurse Caring for a Patient with Congenital Heart Disease Needs to Know Lindsey Justice, DNP, RN, CPNP-AC, Nurse Practitioner, Cardiac Intensive Care Unit, Cincinnati Children’s Hospital Medical Center Justine Mize, MSN, RN, CCRN, CPN, Professional Practice Specialist, Cardiac Intensive Care Unit, Children’s National Health System, Washington, DC Louise Callow, MSN, RN, CPNP, Nurse Practitioner, Pediatric Cardiac Surgery, University of Michigan, CS Mott Children’s Hospital, Ann Arbor, Michigan Mary Rummell, MN, RN, CPNP, CNS, FAHA Clinical Nurse Specialist, Pediatric Cardiology/Cardiac Services, Oregon Health & Science University (Retired) Embryology Occurrence: o Defects of cardiac valves are the most common subtype of cardiac malformations o Account for 25% to 30% of all congenital heart defects o Most costly and relevant CHD o Wise spectrum of congenital defects in tricuspid valve Development of the heart valves occurs during the fourth to eighth weeks of gestation- after tubular heart looping o Walls of the tubular heart consist of an outer lining of myocardium and an inner lining of endocardial cells o Cardiac jelly, extensive extracellular matrix (ECM), separates the two layers o Cardiac jelly expands to form cardiac cushions at the sites of future valves . Outflow track (OT) valves = aortic and pulmonic valves Final valves derived from endothelial-mesenchymal cells with neural crest cells from the brachial arches Valves (Semilunar) have 3 equal cusp-shaped leaflets Aortic valve incorporates coronary arteries . Atrioventricular (AV) valves = mitral and tricuspid Final valves derived entirely from endocardial cushion tissue Leaflet formed without a cusp Two leaflets associated with left ventricle (mitral) Three leaflets associated with right ventricle (tricuspid) Coordinated by complex interplay of: o Genetics o Signaling pathways that regulate cell apoptosis and proliferation o Environmental factors 1 . -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -
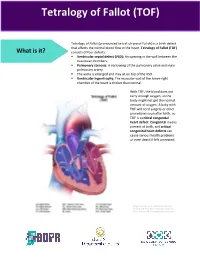
Tetralogy of Fallot (TOF)
Tetralogy of Fallot (TOF) Tetralogy of Fallot (pronounced te-tral-uh-jee of Fal-oh) is a birth defect that affects the normal blood flow in the heart. Tetralogy of Fallot (TOF) What is it? consists of four defects: . Ventricular septal defect (VSD): An opening in the wall between the two lower chambers. Pulmonary stenosis: A narrowing of the pulmonary valve and main pulmonary artery. The aorta is enlarged and may sit on top of the VSD. Ventricular hypertrophy: The muscular wall of the lower-right chamber of the heart is thicker than normal. With TOF, the blood does not carry enough oxygen, so the body might not get the normal amount of oxygen. A baby with TOF will need surgery or other procedures soon after birth, so TOF is a critical congenital heart defect. Congenital means present at birth, and critical congenital heart defects can cause serious health problems or even death if left untreated. Image courtesy of the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities About 1 in every 2,518 babies is born with TOF. That’s about 1,660 How common is it? babies each year in the United States. The cause of TOF for most babies is unknown. There may be many factors that cause TOF, but more research is needed to understand the What causes it? exact cause. TOF can be diagnosed during pregnancy or after. During pregnancy screenings are done to check for birth defects. After birth a doctor will do a physical examination to see whether the baby has blue-colored How is it diagnosed? skin and lips, called cyanosis.