Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patent Ductus Arteriosus
Peer Reviewed Correction of a Canine Left-to-Right Shunting By Connie K. Varnhagen, PhD, RAHT Patent Ductus Arteriosus Scrappy, a 5-year-old, 27-kg, neutered Labrador retriever, presented to the Calgary Animal Referral and Emergency (CARE) Centre in Calgary, Alberta, Canada, for mild exercise intolerance. The owner reported that the normally active dog was “slowing down” and pant- ing excessively even after mild exercise. History time and mucous membrane color were normal. Lung sounds The patient was the runt of its litter, was rejected by its were normal on auscultation. Based on the physical examina- dam, and was hand-fed for the first 5 days. A presumed tion, the differential diagnosis included patent ductus arterio- innocent heart murmur was detected on auscultation at the sus (PDA) or another type of arteriovenous fistula. puppy’s first veterinary examination. Right lateral and ventrodorsal (Figure 1) thoracic radio- At approximately 6 months of age, the patient began graphs revealed cardiomegaly with left atrial and ventricu- experiencing chronic small intestine diarrhea and weight lar enlargement and a prominent pulmonary artery bulge. loss. Over the next 30 months, the dog was diagnosed and Electrocardiography (ECG; Figure 2) demonstrated a tall treated for giardiasis, coccidiosis, small intestinal bacterial R wave (greater than 4.0 mV in lead II compared with a overgrowth, borderline exocrine pancreatic insufficiency, normal parameter of 3.0 mV), indicative of left ventricular and lymphocytic plasmacytic inflammatory bowel disease. enlargement, and normal sinus rhythm. Also at approximately 6 months of age, the patient Echocardiography was performed and was definitive developed a nonseasonal pruritus and dry, brittle haircoat. -
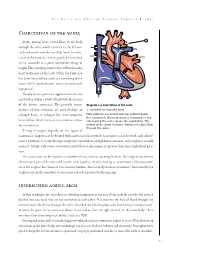
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Echocardiography of the Patent Ductus Arteriosus in Premature Infant
Received: 15 August 2018 | Accepted: 16 October 2018 DOI: 10.1111/chd.12703 SPECIAL ISSUE ARTICLE Echocardiography of the patent ductus arteriosus in premature infant Govinda Paudel MD | Vijaya Joshi MD Pediatric Cardiology, University of Tennessee Health Science Center, Le Abstract Bonheur Children’s Hospital, Memphis, Management of the patent ductus arteriosus (PDA) in the premature infant has been Tennessee a point of controversy for decades as smaller and earlier gestational age infants have Correspondence been surviving. Increasing experience with catheter‐based device closure has gener‐ Govinda Paudel, MD, Pediatric Cardiology, University of Tennessee Health Science ated a new wave of interest in this subject. In this era, echocardiography plays a cen‐ Center, Le Bonheur Children’s Hospital, 49 tral role for collaboration within a multispecialty team. Reliability of echocardiography North Dunlap Street, Memphis, TN, 38103. Email: [email protected] is improved by applying an institutionally derived standard approach to imaging, data collection, and reporting. The key aspects of both the physiology and anatomy of the PDA to distinguish infants that may benefit from intervention are described. KEYWORDS device closure, echocardiography, patent ductus arteriosus, premature infants 1 | INTRODUCTION cardiologists (including interventionist) and neonatologists should agree on the imaging parameters desired and reported. The report Patent ductus arteriosus (PDA) is associated with numerous com‐ summary should have a standard format that tracks serial changes plications in premature babies.1 Hemodynamics of the PDA guide from the previous echo as this is essential to optimize communication. management. But anatomic features have gained importance with Although high‐frequency probes (8‐12 MHz) are most commonly the growing experience in transcatheter device closure of the ductus utilized, lower frequency probes can provide better penetration from in premature infants. -

How to Recognize a Suspected Cardiac Defect in the Neonate
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcare- professionals/education/continuing-medical-nursing-education/neonatal- nursing-education-briefs/ Cardiac defects are commonly seen and are the leading cause of death in the neonate. Prompt suspicion and recognition of congenital heart defects can improve outcomes. An ECHO is not needed to make a diagnosis. Cardiac defects, congenital heart defects, NICU, cardiac assessment How to Recognize a Suspected Cardiac Defect in the Neonate Purpose and Goal: CNEP # 2092 • Understand the signs of congenital heart defects in the neonate. • Learn to recognize and detect heart defects in the neonate. None of the planners, faculty or content specialists has any conflict of interest or will be presenting any off-label product use. This presentation has no commercial support or sponsorship, nor is it co-sponsored. Requirements for successful completion: • Successfully complete the post-test • Complete the evaluation form Date • December 2018 – December 2020 Learning Objectives • Describe the risk factors for congenital heart defects. • Describe the clinical features of suspected heart defects. • Identify 2 approaches for recognizing congenital heart defects. Introduction • Congenital heart defects may be seen at birth • They are the most common congenital defect • They are the leading cause of neonatal death • Many neonates present with symptoms at birth • Some may present after discharge • Early recognition of CHD -

The Patent Ductus Arteriosus (PDA) and the Preterm Baby
12/5/2018 The Patent Ductus Arteriosus (PDA) and the Preterm Baby Tanya Hatfield, RNC - NIC, MSN Neonatal Outreach Educator Objectives ▪ Describe normal cardiac physiology and development ▪ Understand the unique physiologic needs of the preterm infant with a PDA ▪ Define the implications prematurity presents with the cardiac system 2 1 12/5/2018 Normal Cardiovascular Function: Review Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 3 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 Normal Cardiovascular Function: Review Right Left Lungs Body Pulmonary Systemic Artery (PA) Aorta ( Ao ) Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 4 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 2 12/5/2018 Fetal Circulation (2015). Retrieved 29 October 2015, from 5 http://higheredbcs.wiley.com/legacy/college/tortora/0470565101/hearthis_ill/pap13e_ch21_illustr_audio_m p3_am/simulations/hear/fetal_circulation.html Fetal Circulation ▪ Gas exchange is liquid to liquid ▪ Organ of respiration is placenta ▪ High flow, low resistance ▪ Fetal lungs • Low flow, high resistance ▪ PA’s constricted ▪ High right heart and lung pressures ▪ Low left heart pressures ▪ Open fetal -

Artery Umbilical Cord
THE PRACTICAL IMPORTANCE OF THE SINGLE ARTERY UMBILICAL CORD RICHARD F. HNAT Department of Obstetrics and Gynecology, The New York Hospital-Cornell Medical Center, New Yoi [Received 23rd May 1966) Summary. The correlation of single artery umbilical cords with con- genital anomalies is explored in the present study of 4808 consecutive deliveries. There were thirty-eight single artery cords, and six of these were associated with additional foetal abnormalities. An attempt is made to present the correlation in a true perspective by adding these data to other similar studies. Acknowledgment is given to the many retrospective studies, but consecutive delivery series show that a single artery cord is of importance as an abnormal physical finding but not as a screening test to detect occult malformations. INTRODUCTION Recent studies from large obstetrical services have suggested a correlation between the absence of one umbilical artery and congenital anomalies. Although this association had been mentioned before and after the observa¬ tion attributed to Hyrtl, it attracted little attention until the work of Ben- irschke & Brown (1955), a retrospective study of fifty-five placentae and autopsies of newborns from three different institutions. Of these infants, twenty-seven exhibited malformations involving all organ systems. Since 1955 many reports have appeared. Retrospective studies have taken the form of either autopsy material or accumulated cases, usually from different hospitals, added to the experience of the large teaching institution from which the report emanated (Table 1). Prospective studies consist entirely of cord specimens collected from consecutive deliveries, and they have consequently suffered from the small number of cases (Table 2). -

Congenital Cardiovascular Defects
Statistical Fact Sheet 2016 Update Congenital Cardiovascular Defects Congenital cardiovascular defects, also known as congenital heart defects, are structural problems that arise from abnormal formation of the heart or major blood vessels. ICD-9 lists 25 congenital heart defects codes, of which 21 designate specified anatomic or hemodynamic lesions. Defects range in severity from tiny pinholes between chambers that may resolve spontaneously to major malformations that can require multiple surgical procedures before school age and may result in death in utero, in infancy, or in childhood. The common complex defects include the following: Tetralogy of Fallot (TOF) Transposition of the great Trends in age-adjusted death rates attributable to arteries congenital heart defects, 1999 to 2013. Atrioventricular septal de- fects (ASD) Coarctation of the aorta Hypoplastic left heart syn- drome Incidence Congenital heart defects are serious and common condi- tions that have significant impact on morbidity, mortali- ty, and healthcare costs in children and adults. The most commonly report- ed incidence of congenital heart defects in the United States is between 4 and 10 per 1,000, clustering around 8 per 1,000 live births. Continental variations in birth prevalence have been reported, from 6.9 per 1000 births in Europe to 9.3 per 1000 in Asia. An estimated minimum of 40,000 infants are expected to be affected each year in the United States. Of these, about 25%, or 2.4 per 1,000 live births, require invasive treatment in the first year of life. Prevalence It is estimated that there are an estimated 650,000 to 1.3 million children and adults living with con- genital heart disease in the United States. -

Usefulness Ofcontinuous Positive Airway Pressure in Differential
Arch Dis Child: first published as 10.1136/adc.53.6.456 on 1 June 1978. Downloaded from Archives of Disease in Childhood, 1978, 53, 456-460 Usefulness of continuous positive airway pressure in differential diagnosis of cardiac from pulmonary cyanosis in newborn infants P. SYAMASUNDAR RAO, BRENDA L. MARINO, AND ALEX F. ROBERTSON III From the Department of Pediatrics, Sections of Pediatric Cardiology and Neonatology, Medical College of Georgia, Augusta, Georgia, USA SUMMARY Differential diagnosis of cyanosis in the neonate is difficult and cardiac catheterisa- tion may be required for a correct diagnosis. It has been suggested that the response of Pao2 to continuous positive airway pressure (CPAP) with 100% oxygen may be useful. The purpose of this study was to test further this hypothesis by studying all neonates investigated for cyanosis with a Pao2 -50 torr in 0 8 to 1 .0 F1o2. Arterial blood samples were obtained in an F1o2 of 0 21-0 .4 and 0 8-1 .0, and in an F1O2 of 0 8-1 0 with 8-10 cm CPAP, and were analysed for Pao2, Paco2, and pH, bicarbonate being calculated. The final diagnoses were congenital heart disease (CHD) 21 cases, pulmonary parenchymal disease (PD) 10 cases, and persistent fetal circulation (PFC) 3 cases. No significant difference in pH, bicarbonate, or Paco2 was observed among the three groups or with CPAP. In the CHD and PFC infants CPAP produced no significant change in Pao2. In the PD babies Pao2 increased by an average of 33 torr (P<0 05). Despite thus attaining statistical significance 2 PD infants had no increase in Pao2 with CPAP. -

Pharmacy Policy Statement
PHARMACY POLICY STATEMENT Ohio Medicaid DRUG NAME Synagis (palivizumab) BILLING CODE 90378 (1 unit = 1 vial) BENEFIT TYPE Medical SITE OF SERVICE ALLOWED Office/Outpatient Hospital/Home COVERAGE REQUIREMENTS Prior Authorization Required (Preferred Product) QUANTITY LIMIT— 1 vial per month (max 5 during respiratory syncytial virus season) LIST OF DIAGNOSES CONSIDERED NOT Click Here MEDICALLY NECESSARY Synagis (palivizumab) is a preferred product and will only be considered for coverage under the medical/pharmacy benefit when the following criteria are met: Members must be clinically diagnosed with one of the following disease states and meet their individual criteria as stated. PREVENTION OF RESPIRATORY TRACT DISEASE CAUSED BY RESPIRATORY SYNCYTIAL VIRUS (RSV) For initial authorization: 1. Request must be made during the RSV season (November 1st through March 31st) AND initiation of injections should be timed with the onset of laboratory confirmed cases of RSV activity in the community, no earlier than November 1, 2017; AND 2. Member is < 12 months old at the beginning of the RSV season AND meet one of the following criteria (chart notes must be provided to support evidence): a) Member was born < 29 weeks, 0 days’ gestation; b) Member has Chronic Lung Disease (CLD) of prematurity (defined as gestational age <32 weeks, 0 days and a requirement for >21% oxygen for at least the first 28 days after birth); c) Member has hemodynamically significant Congenital Heart Disease (CHD) with one or more of the following: i) Acyanotic heart disease (e.g. atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), etc.), AND member is receiving medication to control congestive heart failure (CHF) AND will require cardiac surgical procedures; ii) Moderate to severe pulmonary hypertension; iii) Cyanotic heart defect (e.g. -

Congenital Heart Disease Parent FAQ
Congenital Heart Disease Parent FAQ achd.stanfordchildrens.org | achd.stanfordhealthcare.org About Congenital Heart Disease What is congenital heart disease? Congenital heart disease, also called congenital heart defect (CHD), is a heart problem that a baby is born with. When the heart forms in the womb, it develops incorrectly and does not work properly, which changes how the blood flows through the heart. What causes congenital heart defects? In most cases, there is no clear cause. It can be linked to something out of the ordinary happening during gestation, including a viral infection or exposure to environmental factors. Or, it may be linked to a single gene defect or chromosome abnormalities. How common is CHD in the United States among children? Congenital heart defects are the most common birth defects in children in the United States. Approximately 1 in 100 babies are born with a heart defect. What are the most common types of congenital heart defects in children? In general, CHDs disrupt the flow of blood in the heart as it passes to the lungs or to the body. The most common congenital heart defects are abnormalities in the heart valves or a hole between the chambers of the heart (ventricles). Examples include ventricular septal defect (VSD), atrial septal defect (ASD), and bicuspid aortic valve. At the Betty Irene Moore Children’s Heart Center at Stanford Children’s Health, we are known across the nation and world for treating some of the most complex congenital heart defects with outstanding outcomes. Congenital Heart Disease Parent FAQ | 2 Is CHD preventable? In some cases, it could be preventable. -

Review Article Congenital Heart Diseases
KYAMC Journal Vol. 9, No.1, April 2018 Review Article Congenital heart diseases: A review of echocardiogram records Md. Saiful Islam1, Md. Moniruzzaman2 Abstract Congenital heart defect (CHD) means an anatomic malformation of the heart or great vessels which occurs during intrauterine development, irrespective of the age at presentation. They can disrupt the normal blood flow through the heart. The blood flow can slow down, go in the wrong direction or to the wrong place, or be blocked completely. Broadly congenital heart defects can be acyanotic and cyanotic. We have reviewed retrospectively from echocardiogram record nearly two years of period & collected total 404 patients with congenital heart defects. Among them 329 (81.43%) was acyanotic and 75 (18.57%) was cyanotic congenital defects with variety of diagnosis. Ventricular septal defect was the most common acyanotic heart defect and Tetralogy of Fallot was the most common cyanotic heart defect. There was no significant gender deference. Keywords: Acyanotic, Congenital heart disease, Cyanotic. Date of received: 11. 11. 2017 Date of acceptance: 05. 01. 2018 Introduction known. The majority of the defects can be explained by Congenital heart defects (CHD) are reported in almost 1% of multifactorial inheritance hypothesis which states that a live births, and about half of these children need medical or predisposed fetus, when exposed to a given environmental surgical management in infancy1. In the first decade, a further trigger, to which the fetus is sensitive during the critical period 25% require surgery to maintain or improve their life1. Only of cardiac morphogenesis may develop the disease5. A variety 10% survive to adolescence without specific treatment.