Tetralogy of Fallot.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Recognize a Suspected Cardiac Defect in the Neonate
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcare- professionals/education/continuing-medical-nursing-education/neonatal- nursing-education-briefs/ Cardiac defects are commonly seen and are the leading cause of death in the neonate. Prompt suspicion and recognition of congenital heart defects can improve outcomes. An ECHO is not needed to make a diagnosis. Cardiac defects, congenital heart defects, NICU, cardiac assessment How to Recognize a Suspected Cardiac Defect in the Neonate Purpose and Goal: CNEP # 2092 • Understand the signs of congenital heart defects in the neonate. • Learn to recognize and detect heart defects in the neonate. None of the planners, faculty or content specialists has any conflict of interest or will be presenting any off-label product use. This presentation has no commercial support or sponsorship, nor is it co-sponsored. Requirements for successful completion: • Successfully complete the post-test • Complete the evaluation form Date • December 2018 – December 2020 Learning Objectives • Describe the risk factors for congenital heart defects. • Describe the clinical features of suspected heart defects. • Identify 2 approaches for recognizing congenital heart defects. Introduction • Congenital heart defects may be seen at birth • They are the most common congenital defect • They are the leading cause of neonatal death • Many neonates present with symptoms at birth • Some may present after discharge • Early recognition of CHD -

Reoperation for Tricuspid Regurgitation After Total Correction of Tetralogy of Fallot
Original Reoperation for Tricuspid Regurgitation after Total Article Correction of Tetralogy of Fallot Yoshikazu Hachiro, MD, Nobuyuki Takagi, MD, Tetsuya Koyanagi, MD, and Tomio Abe, MD Background: The aim of this study is to review the outcome of reoperation for severe tricus- pid regurgitation after repair of tetralogy of Fallot . Methods: Between 1972 and 2000, 12 patients underwent reoperation on the tricuspid valve after total correction of tetralogy of Fallot. The mean age at the time of reoperation was 17 years (range, 1 to 39 years). The mean interval between the initial correction and the reoperation was 7.8 years (range, 10 days to 19 years). The functional class was New York Heart Association class II in 2 patients and class III or IV in 10. Six patients underwent tricuspid valve repair, and the others underwent tricuspid valve replacement. Results: Hospital mortality was 16.7% (2/12). Three patients (30%, 3/10) required a second reoperation 1.6, 9.2, and 15.6 years after the most recent reoperation with no deaths. The rea- sons for second reoperation were failure of the tricuspid valve repair in two and a thrombosed valve in one. There were two late deaths. Mean overall event-free actuarial survival at 10 years was 46.3%. Conclusion: Reoperation for severe tricuspid regurgitation after total correction of tetralo- gy of Fallot was associated with a high operative mortality and disappointing long-term results. Tricuspid regurgitation after corrective surgery for tetralogy of Fallot must be diag- nosed promptly and cured, as tolerance is poor because of postoperative right ventricular insufficiency. -
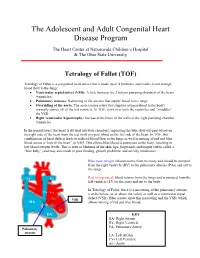
Tetralogy of Fallot (TOF)
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children’s Hospital & The Ohio State University Tetralogy of Fallot (TOF) Tetralogy of Fallot is a congenital heart defect that is made up of 4 problems and results in not enough blood flow to the lungs: • Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles) • Pulmonary stenosis: Narrowing of the arteries that supply blood to the lungs • Overriding of the aorta: The aorta (major artery that supplies oxygen blood to the body) normally comes off of the left ventricle. In TOF, it sits over both the ventricles and “straddles” the VSD. • Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) In the normal heart, the heart is divided into four chambers, separating the blue (low-oxygen) blood on the right side of the heart from the red (with oxygen) blood on the left side of the heart. In TOF, this combination of heart defects leads to reduced blood flow to the lungs as well as mixing of red and blue blood across a “hole in the heart” or VSD. This allows blue blood to pump out to the body, resulting in low blood oxygen levels. This is seen as blueness of the skin, lips, fingernails and tongue (often called a “blue baby”) and may also result in poor feeding, growth problems, and activity intolerance. Blue (low-oxygen) blood returns from the body and should be pumped from the right ventricle (RV) to the pulmonary arteries (PAs) and out to Aorta the lungs. -

Congenital Cardiovascular Defects
Statistical Fact Sheet 2016 Update Congenital Cardiovascular Defects Congenital cardiovascular defects, also known as congenital heart defects, are structural problems that arise from abnormal formation of the heart or major blood vessels. ICD-9 lists 25 congenital heart defects codes, of which 21 designate specified anatomic or hemodynamic lesions. Defects range in severity from tiny pinholes between chambers that may resolve spontaneously to major malformations that can require multiple surgical procedures before school age and may result in death in utero, in infancy, or in childhood. The common complex defects include the following: Tetralogy of Fallot (TOF) Transposition of the great Trends in age-adjusted death rates attributable to arteries congenital heart defects, 1999 to 2013. Atrioventricular septal de- fects (ASD) Coarctation of the aorta Hypoplastic left heart syn- drome Incidence Congenital heart defects are serious and common condi- tions that have significant impact on morbidity, mortali- ty, and healthcare costs in children and adults. The most commonly report- ed incidence of congenital heart defects in the United States is between 4 and 10 per 1,000, clustering around 8 per 1,000 live births. Continental variations in birth prevalence have been reported, from 6.9 per 1000 births in Europe to 9.3 per 1000 in Asia. An estimated minimum of 40,000 infants are expected to be affected each year in the United States. Of these, about 25%, or 2.4 per 1,000 live births, require invasive treatment in the first year of life. Prevalence It is estimated that there are an estimated 650,000 to 1.3 million children and adults living with con- genital heart disease in the United States. -
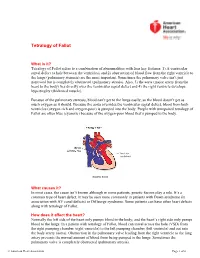
Before Reading the Specific Defect
Tetralogy of Fallot What is it? Tetralogy of Fallot refers to a combination of abnormalities with four key features: 1) A ventricular septal defect (a hole between the ventricles) and 2) obstruction of blood flow from the right ventricle to the lungs (pulmonary stenosis) are the most important. Sometimes the pulmonary valve isn’t just narrowed but is completely obstructed (pulmonary atresia). Also, 3) the aorta (major artery from the heart to the body) lies directly over the ventricular septal defect and 4) the right ventricle develops hypertrophy (thickened muscle). Because of the pulmonary stenosis, blood can’t get to the lungs easily, so the blood doesn’t get as much oxygen as it should. Because the aorta overrides the ventricular septal defect, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body. People with unrepaired tetralogy of Fallot are often blue (cyanotic) because of the oxygen-poor blood that’s pumped to the body. What causes it? In most cases, the cause isn’t known although in some patients, genetic factors play a role. It’s a common type of heart defect. It may be seen more commonly in patients with Down syndrome (in association with AV canal defects) or DiGeorge syndrome. Some patients can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a patient with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). -
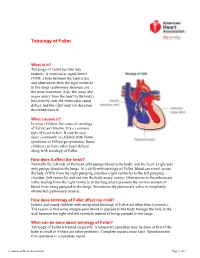
Tetralogy of Fallot
Tetralogy of Fallot What is it? Tetralogy of Fallot has four key features. A ventricular septal defect (VSD; a hole between the ventricles) and obstruction from the right ventricle to the lungs (pulmonary stenosis) are the most important. Also, the aorta (the major artery from the heart to the body) lies directly over the ventricular septal defect, and the right ventricle develops thickened muscle. What causes it? In most children, the cause of tetralogy of Fallot isn’t known. It’s a common type of heart defect. It may be seen more commonly in children with Down syndrome or DiGeorge syndrome. Some children can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a child with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). Obstruction in the pulmonary valve leading from the right ventricle to the lung artery prevents the normal amount of blood from being pumped to the lungs. Sometimes the pulmonary valve is completely obstructed (pulmonary atresia). How does tetralogy of Fallot affect my child? Infants and young children with unrepaired tetralogy of Fallot are often blue (cyanotic). The reason is that some oxygen-poor blood is pumped to the body through the hole in the wall between the right and left ventricle instead of being pumped to the lungs. -

Congenital Heart Disease Parent FAQ
Congenital Heart Disease Parent FAQ achd.stanfordchildrens.org | achd.stanfordhealthcare.org About Congenital Heart Disease What is congenital heart disease? Congenital heart disease, also called congenital heart defect (CHD), is a heart problem that a baby is born with. When the heart forms in the womb, it develops incorrectly and does not work properly, which changes how the blood flows through the heart. What causes congenital heart defects? In most cases, there is no clear cause. It can be linked to something out of the ordinary happening during gestation, including a viral infection or exposure to environmental factors. Or, it may be linked to a single gene defect or chromosome abnormalities. How common is CHD in the United States among children? Congenital heart defects are the most common birth defects in children in the United States. Approximately 1 in 100 babies are born with a heart defect. What are the most common types of congenital heart defects in children? In general, CHDs disrupt the flow of blood in the heart as it passes to the lungs or to the body. The most common congenital heart defects are abnormalities in the heart valves or a hole between the chambers of the heart (ventricles). Examples include ventricular septal defect (VSD), atrial septal defect (ASD), and bicuspid aortic valve. At the Betty Irene Moore Children’s Heart Center at Stanford Children’s Health, we are known across the nation and world for treating some of the most complex congenital heart defects with outstanding outcomes. Congenital Heart Disease Parent FAQ | 2 Is CHD preventable? In some cases, it could be preventable. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -
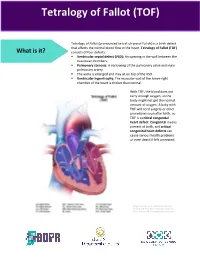
Tetralogy of Fallot (TOF)
Tetralogy of Fallot (TOF) Tetralogy of Fallot (pronounced te-tral-uh-jee of Fal-oh) is a birth defect that affects the normal blood flow in the heart. Tetralogy of Fallot (TOF) What is it? consists of four defects: . Ventricular septal defect (VSD): An opening in the wall between the two lower chambers. Pulmonary stenosis: A narrowing of the pulmonary valve and main pulmonary artery. The aorta is enlarged and may sit on top of the VSD. Ventricular hypertrophy: The muscular wall of the lower-right chamber of the heart is thicker than normal. With TOF, the blood does not carry enough oxygen, so the body might not get the normal amount of oxygen. A baby with TOF will need surgery or other procedures soon after birth, so TOF is a critical congenital heart defect. Congenital means present at birth, and critical congenital heart defects can cause serious health problems or even death if left untreated. Image courtesy of the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities About 1 in every 2,518 babies is born with TOF. That’s about 1,660 How common is it? babies each year in the United States. The cause of TOF for most babies is unknown. There may be many factors that cause TOF, but more research is needed to understand the What causes it? exact cause. TOF can be diagnosed during pregnancy or after. During pregnancy screenings are done to check for birth defects. After birth a doctor will do a physical examination to see whether the baby has blue-colored How is it diagnosed? skin and lips, called cyanosis. -

Transcatheter Device Closure of Atrial Septal Defect in Dextrocardia with Situs Inversus Totalis
Case Report Nepalese Heart Journal 2019; Vol 16(1), 51-53 Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis Kiran Prasad Acharya1, Chandra Mani Adhikari1, Aarjan Khanal2, Sachin Dhungel1, Amrit Bogati1, Manish Shrestha3, Deewakar Sharma1 1 Department of Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal 2 Department of Internal Medicine, Kathmandu Medical College, Kathmandu,Nepal 3 Department of Pediatric Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal Corresponding Author: Chandra Mani Adhikari Department of Cardiology Shahid Gangalal National Heart Centre Kathmandu, Nepal Email: [email protected] Cite this article as: Acharya K P, Adhikari C M, Khanal A, et al. Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis. Nepalese Heart Journal 2019; Vol 16(1), 51-53 Received date: 17th February 2019 Accepted date: 16th April 2019 Abstract Only few cases of Device closure of atrial septal defect in dextrocardia with situs inversus totalis has been reported previously. We present a case of a 36 years old male, who had secundum type of atrial septal defect in dextrocardia with situs inversus totalis. ASD device closure was successfully done. However, we encountered few technical difficulties in this case which are discussed in this case review. Keywords: atrial septal defect; dextrocardia; transcatheter device closure, DOI: https://doi.org/10.3126/njh.v16i1.23901 Introduction There are only two case reported of closure of secundum An atrial septal defect (ASD) is an opening in the atrial ASD associated in patients with dextrocardia and situs inversus septum, excluding a patent foramen ovale.1 ASD is a common totalis. -

Congenital Heart Disease: Recognition and Treatment Options – Anna Gelzer
CONGENITAL HEART DISEASE: RECOGNITION AND TREATMENT OPTIONS – ANNA GELZER Prevalence and Frequency Congenital heart disease in dogs: ≤ 1% (Canine clinic population of U Penn 1992) Cats estimated < 0.2% Prevalence likely underestimated (perinatal death, no murmur) Small % of cardiac disease overall, but most common in animals < 1 y old Common congenital cardiac defects in dogs (in the order of frequency): Subaortic stenosis (SAS) Pulmonic stenosis (PS) Patent ductus arteriosus (PDA) Tricuspid valve dysplasia (TVD) Ventricular septal defect (VSD) Tetralogy of Fallot (TOF) Persistent right aortic arch (PRAA) Atrial septal defects (ASD) Mitral valve dysplasia (MVD) Persistent left carnial venal cava Congenital cardiac defects in cats (in the order of frequency): Mitral valve dysplasia (MVD) Ventricular septal defect (VSD) Endocardial cushion defect (ASD+VSD) Patent ductus arteriosus (PDA) Aortic stenosis (SAS) Tetralogy of Fallot (TOF) Pulmonic stenosis (PS) Atrial septal defects (ASD) Tricuspid valve dysplasia (TVD) Physical exam finding in animals with most common congenital heart disease: The simple congenital heart defects are normally identified by the presence of a heart murmur. From the clinical examination, by localizing the heart murmur, identifying its radiation, assessing the precordial impulse and the peripheral pulse, a differential diagnosis list can be drawn up. The table summarizes the findings for some of the common defects identified in dogs and cats. Note, for these simple defects, mucus membrane color is normal (pink). -

Association of Hand Anomalies with Congenital Heart Lesions in Mentally Retarded, State- Institutionalized Patients Marian Grace Jordison Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1968 Association of hand anomalies with congenital heart lesions in mentally retarded, state- institutionalized patients Marian Grace Jordison Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Jordison, Marian Grace, "Association of hand anomalies with congenital heart lesions in mentally retarded, state-institutionalized patients" (1968). Yale Medicine Thesis Digital Library. 2756. http://elischolar.library.yale.edu/ymtdl/2756 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE MEDICAL LIBRARY 9002 01065 5752 seek ^ j IptxL-ffij y Li: Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/associationofhanOOjord ASSOCIATION OF HAND ANOMALIES WITH CONGENITAL HEART LESIONS IN MENTALLY RETARDED, STATE- INSTITUTIONALIZED PATIENTS Marian Grace Jordison B.A. Stanford University, 1964 A Thesis Submitted in Partial Fulfillment of the Requirement for the Degree of Doctor of Medicine Department of Epidemiology & Public Health Yale University School of Medicine April, 1968 y/3. TABLE OF CONTENTS PAGE Introduction. 1 Review of the literature. 3 I. Holt-Oram syndrome. 3 II. Epidemiology of congenital malformations. 6 A. Incidence. 6 B.